NIST967A
Creatinine in frozen human serum
NIST® SRM® 967a
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
41116107
Nomenclature NACRES :
NA.24
Produits recommandés
Qualité
certified reference material
Niveau de qualité
Forme
liquid
Conditionnement
pkg of set(4) (2 ea conc)
Fabricant/nom de marque
NIST®
Technique(s)
mass spectrometry (MS): suitable
Application(s)
clinical testing
Format
matrix material
Description générale
This human blood serum creatinine is a standard reference material (SRM) that consists of four stoppered vials of frozen human serum, two vials each at two different creatinine concentration levels. Each vial contains 1.0 mL of human serum.
SRM 967A_cert
SRM 967A_SDS
SRM 967A_cert
SRM 967A_SDS
Application
This human blood serum creatinine is intended primarily for use in evaluating the accuracy of procedures for the determination of creatinine in human serum. It is also meant for use in validating working or secondary reference materials.
Autres remarques
- For more information on storage, usage, and expiry refer to the NIST certificate.
- Notes for biomaterials, disposal, and transport information are available in SDS.
Certified for the analytes listed below.
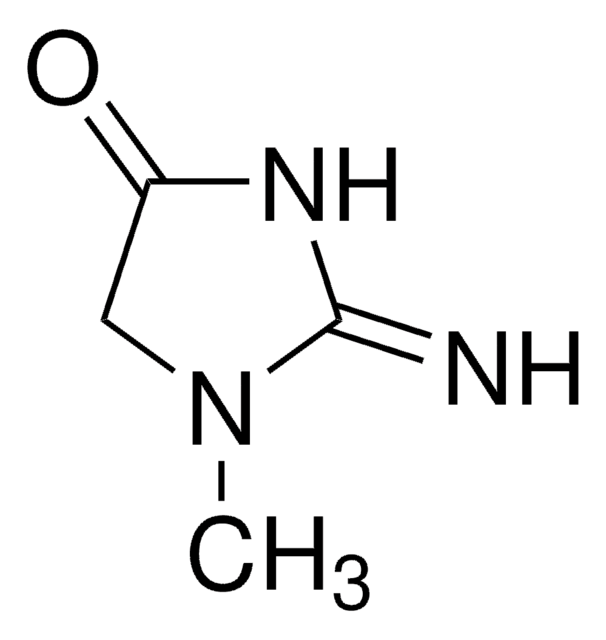
Creatinine
See certificate for values and more details at nist.gov/SRM.
Creatinine
See certificate for values and more details at nist.gov/SRM.
Notes préparatoires
- The serum is shipped frozen on dry ice and, upon receipt, should be stored frozen until ready for use.
- Vials of the SRM to be analyzed should be removed from the freezer and allowed to stand at room temperature (20 °C to 25 °C) until thawed.
Informations légales
NIST is a registered trademark of National Institute of Standards and Technology
SRM is a registered trademark of National Institute of Standards and Technology
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Lot/Batch Number
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
W Greg Miller et al.
Advances in chronic kidney disease, 25(1), 7-13 (2018-03-04)
In 2002, the Kidney Disease Outcomes Quality Initiative guidelines for identifying and treating CKD recommended that clinical laboratories report estimated glomerular filtration rate (eGFR) with every creatinine result to assist clinical practitioners to identify people with early-stage CKD. At that
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique