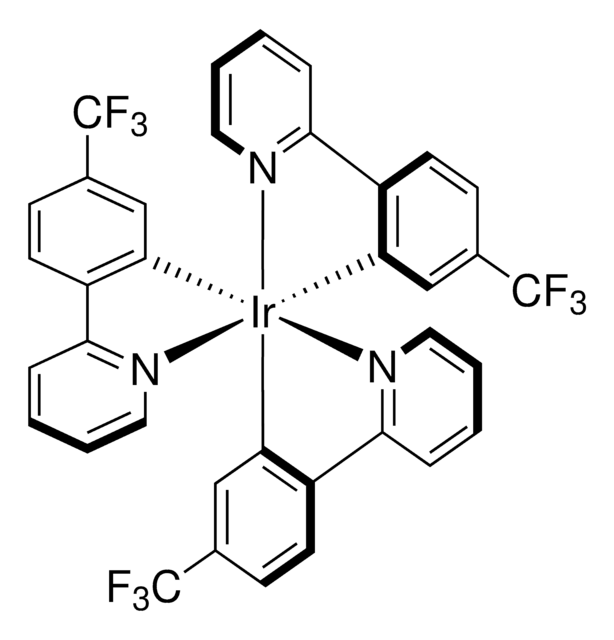
902217
Ir[dFFppy]2-(4,4′-dCF3bpy)PF6
≥95%
Synonyme(s) :
Ir photocatalyst, Photocatalyst
About This Item
Produits recommandés
Pureté
≥95%
Forme
powder or crystals
Pertinence de la réaction
core: iridium
reagent type: catalyst
reaction type: Photocatalysis
Pf
>300 °C
Activation du photocatalyseur
450 nm
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
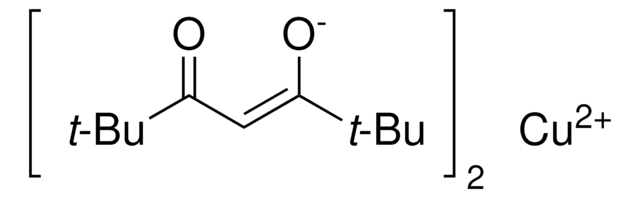
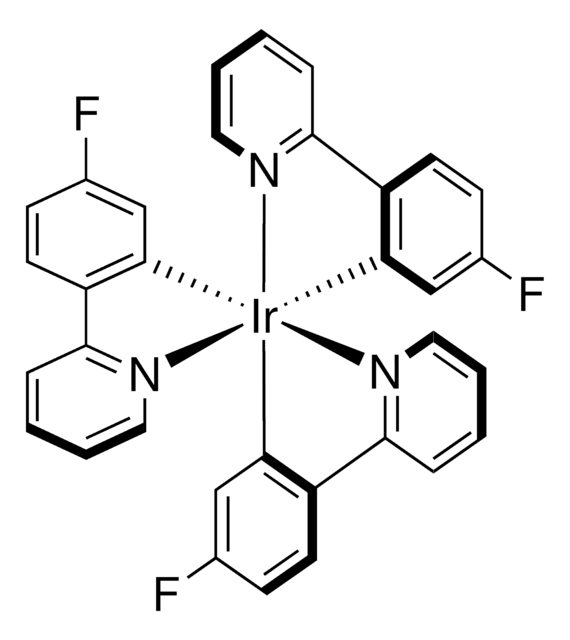
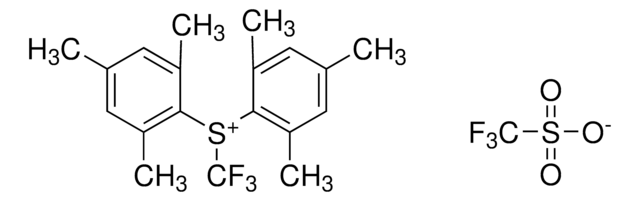
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)

![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![Ir[dFMeppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/355/743/1aef4eef-372d-4f1d-8904-08df7c4fd417/640/1aef4eef-372d-4f1d-8904-08df7c4fd417.png)

![[Ir(p-F(Me)ppy)2-(4,4′-dtbbpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/231/079/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4/640/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4.png)
![Ir[p-F(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/189/186/7badaac3-82af-4109-aab5-dea3a3aa916d/640/7badaac3-82af-4109-aab5-dea3a3aa916d.png)
![[Ru(bpz)3][PF6]2 95%](/deepweb/assets/sigmaaldrich/product/structures/317/925/f0ef928e-bbea-4535-abe6-dda0bc28d32a/640/f0ef928e-bbea-4535-abe6-dda0bc28d32a.png)