517127
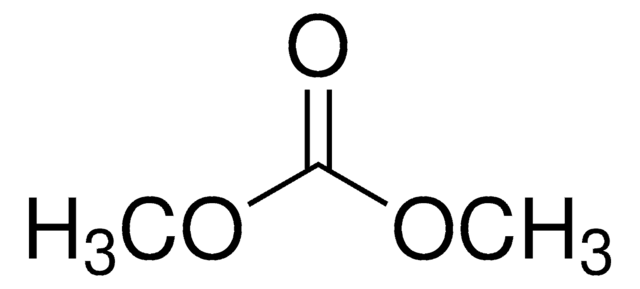
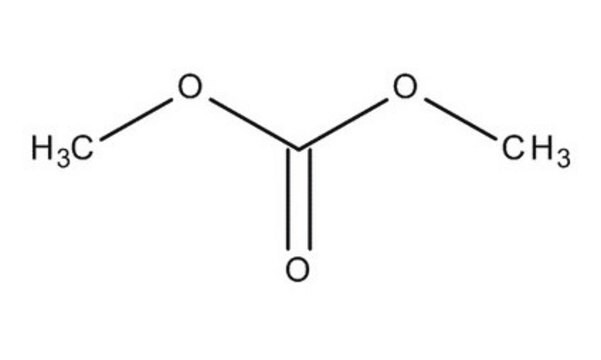
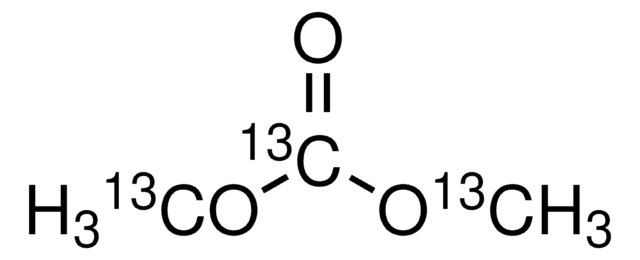
Dimethyl carbonate
anhydrous, ≥99%
Synonyme(s) :
DMC, Carbonic acid dimethyl ester
About This Item
Produits recommandés
Qualité
anhydrous
Niveau de qualité
Densité de vapeur
3.1 (vs air)
Pression de vapeur
18 mmHg ( 21.1 °C)
Pureté
≥99%
Forme
liquid
Limite d'explosivité
4.22-12.87 % (lit.)
Caractéristiques du produit alternatif plus écologique
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
<0.002% water
<0.005% water (100mL pkg)
Couleur
APHA: <50
Indice de réfraction
n20/D 1.368 (lit.)
Point d'ébullition
90 °C (lit.)
Pf
2-4 °C (lit.)
Densité
1.069 g/mL at 25 °C (lit.)
Autre catégorie plus écologique
Chaîne SMILES
O=C(OC)OC
InChI
1S/C3H6O3/c1-5-3(4)6-2/h1-2H3
Clé InChI
IEJIGPNLZYLLBP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is a Greener alternative to conventional solvents and chemicals. Click here for more information.
Application
Caractéristiques et avantages
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Flam. Liq. 2
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
60.8 °F - closed cup
Point d'éclair (°C)
16 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Contenu apparenté
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique