5154
Silk Fibroin
from domesticated Bombyx mori silkworm, liquid, 50 mg/mL, suitable for cell culture, used for 3D scaffolds
Synonyme(s) :
Silk Fibroin Solution
About This Item
Produits recommandés
Nom du produit
Silk, Fibroin Solution, 50 mg/mL
Source biologique
domesticated Bombyx mori silkworm
Niveau de qualité
Forme
solution
Poids mol.
avg mol wt 100 kDa
Concentration
50 mg/mL
~50 mg/mL
Application(s)
clinical testing
Conditions d'expédition
dry ice
Température de stockage
−70°C
Description générale
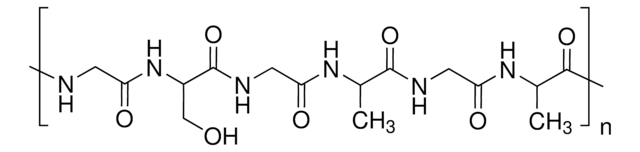
Fibroin protein is the major structural component of the silkworm′s cocoon fiber. Fibroin offers great potential for use in medically related applications due to the high degree of biocompatibility and lack of immune response when implanted within the body. The silk fiber is solubilized into an aqueous fibroin solution, which can then be used as an additive in culture or for producing 3D scaffolds for tissue-engineering related studies.
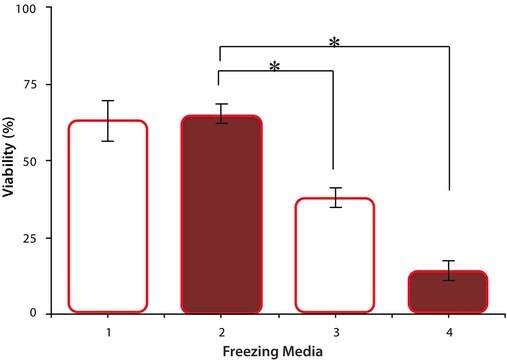
As with traditional tissue-engineering approaches, the silk scaffolds are typically seeded in vitro with a specific cell type as most cells will adhere to fibroin protein, and then cultured over time to mimic tissue architecture. It has been shown that the silk fibroin protein can be degraded a number of naturally occurring proteolytic enzymes, and is thus a biologically active scaffold unlike other synthetic materials. As a result the silk scaffold material is degraded and remodeled through similar physiological pathways in the body. Silk fibroin protein is composed of both non-essential and essential amino acids, with a particular concentration of alanine and glycine present, and these amino acids are then reabsorbed by the surrounding cells for new tissue regeneration. This is important as silk degradation products do not collect in the local environment to induce a toxicity which is commonly associated with other synthetic and naturally occurring biomaterials.
The ability to produce a variety of forms and formats scaffold types (e.g. coatings, films, sponges, hydrogels, electro-spun fibers, micro/nanospheres, etc.) offers a number of advantages over other biopolymer systems like collagen, chitosan, and alginate that have less variety in processing choices. The silk material properties can then be modified through a variety of processing techniques to change degradation rate, hydrophobicity/hydrophilicity, transparency, mechanical strength, porosity, oxygen permeability, and thermal stability. In this regard, silk proteins represent a class of biopolymers with definable material properties for a given application.
If culturing cells using this product, measures should be taken to maintain sterility of cultures such as use of antibiotics.
Notes préparatoires
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique