PF063
MMP-3, Proenzyme, Human, Recombinant
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Pureté
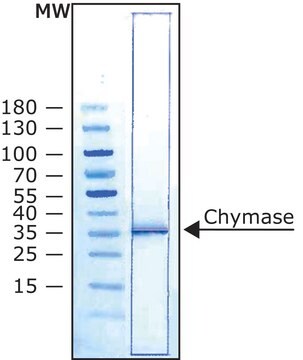
≥95% (SDS-PAGE)
Niveau de qualité
Forme
lyophilized
Fabricant/nom de marque
Calbiochem®
Conditions de stockage
OK to freeze
avoid repeated freeze/thaw cycles
Solubilité
water: soluble
Conditions d'expédition
wet ice
Température de stockage
−70°C
Description générale
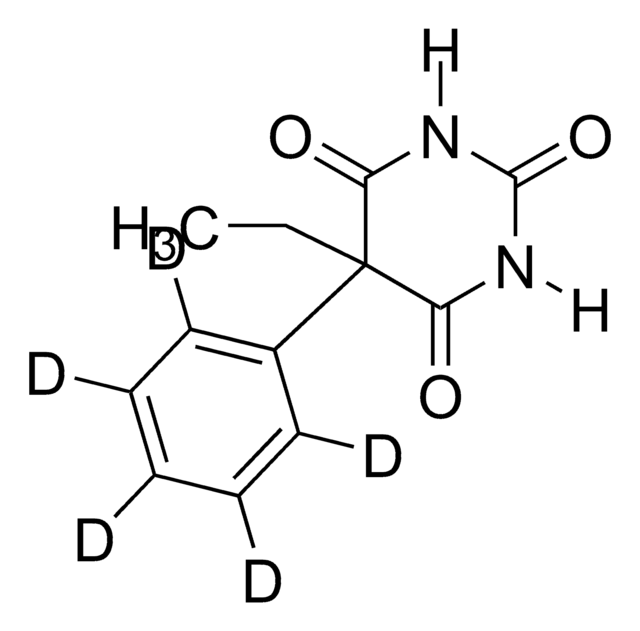
Recombinant, human pro-MMP-3 purified from cell culture supernatant. May be used as a positive control or standard for zymographic analysis, or substrate assay. Requires activation for immunoblotting, prior to use. M.W. 57000/58000.
Recombinant, human pro-MMP-3 purified from cell culture supernatant. May be used as a positive control or standard for zymographic analysis, or substrate assay. Requires activation for immunoblotting, prior to use. M.W. 57000/58000.
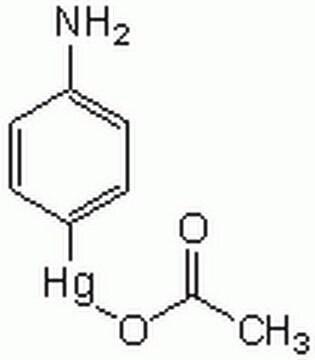
Matrix metalloproteinases (MMPs) are a family of enzymes that are responsible for the degradation of extracellular matrix components such as collagen, laminin and proteoglycans. In addition to sequence homology, all MMPs share the following characteristics: the catalytic mechanism is dependent upon a zinc ion at the active center, they cleave one or more extracellular matrix components, they are secreted as zymogens which are activated by removal of an approximately 10 kDa segment from the N terminus and they are inhibited by tissue inhibitor of metalloproteinases (TIMP). These enzymes are involved in normal physiological processes such as embryogenesis and tissue remodeling and may play an important role in angiogenesis, arthritis, periodontitis, and metastasis. Matrix metalloproteinase-3 (MMP-3) also known as stromelysin-1 and transin (EC 3.4.24.17) cleaves a number of substrates including cartilage proteoglycan, collagen types II, III, IV, V and IX, fibronectin, laminin, and can activate MMP 1. MMP-3 is secreted as ~57 and ~59 kDa proenzymes and can be activated in vitro by organomercurials (e.g., 4 aminophenylmercuric acetate, APMA) and in vivo by proteases via intermediate forms to a 45 kDa active MMP 3 enzyme. Further autolysis to a ~28 kDa form can also occur. MMP-3 is thought to play an important role in pathophysiological degradation processes associated with conditions such as rheumatoid arthritis and cancer cell invasion.
Matrix metalloproteinases (MMPs) are a family of enzymes that are responsible for the degradation of extracellular matrix components such as collagen, laminin and proteoglycans. In addition to sequence homology, all MMPs share the following characteristics: the catalytic mechanism is dependent upon a zinc ion at the active center, they cleave one or more extracellular matrix components, they are secreted as zymogens which are activated by removal of an approximately 10 kDa segment from the N terminus and they are inhibited by tissue inhibitor of metalloproteinases (TIMP). These enzymes are involved in normal physiological processes such as embryogenesis and tissue remodeling and may play an important role in angiogenesis, arthritis, periodontitis, and metastasis. Matrix metalloproteinase-3 (MMP-3) also known as stromelysin-1 and transin (EC 3.4.24.17) cleaves a number of substrates including cartilage proteoglycan, collagen types II, III, IV, V and IX, fibronectin, laminin, and can activate MMP 1. MMP-3 is secreted as ~57 and ~59 kDa proenzymes and can be activated in vitro by organomercurials (e.g., 4 aminophenylmercuric acetate, APMA) and in vivo by proteases via intermediate forms to a 45 kDa active MMP 3 enzyme. Further autolysis to a ~28 kDa form can also occur. MMP-3 is thought to play an important role in pathophysiological degradation processes associated with conditions such as rheumatoid arthritis and cancer cell invasion.
Application
Immunoblotting (see comments)
Substrate Cleavage Assay (see comments)
Zymography (see comments)
Substrate Cleavage Assay (see comments)
Zymography (see comments)
Avertissement
Toxicity: Standard Handling (A)
Forme physique
Lyophilized from 100 mM NaCl, 50 mM HEPES, pH 7.3.
Reconstitution
Following reconstitution, aliquot into siliconized vials and freeze (-70°C).
Remarque sur l'analyse
The activity of proenzyme MMP 3 was measured by substrate cleavage assay using 0.5 mM thiopeptiolide (Ac-Pro-Leu-Gly-S-Leu-Leu-Gly-Oet) as a substrate. The activity was also assessed by degradation of a peptide substrate (DNP-PYAYWMR) using activated MMP-3 as measured by HPLC.
Autres remarques
Proenzyme MMP-3 may be used as a positive control or standard for immunoblotting, zymographic analysis, or substrate cleavage assays. 0.5 μg/lane was used for SDS-PAGE and immunoblotting. For zymography with casein 1μg/lane of Proenzyme MMM-3 or activated MMP-3 was used. Proenzyme MMP-3 can be activated in vitro by incubation in 50 mM Tris, pH 7.5, containing 0.05% Triton-X-100, 5 mM CaCl2 and 1 mM 4 aminophenyl mercuric acetate (APMA) for 2-4 hours at 37°C. To dissolve APMA, make a 10 mM stock solution in 0.05 M NaOH. Approximately 90% of proenzyme MMP-3 is activated with a 4 hour incubation at 37°C using 1 mM APMA.
Informations légales
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
D Cottam et al.
International journal of oncology, 2(6), 861-872 (1993-06-01)
In order for tumor cells to colonise secondary organs and tissues it is necessary for them to be able to complete all the essential steps of the metastatic cascade. We discuss here some of the important aspects of this process
J F Woessner
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 5(8), 2145-2154 (1991-05-11)
Matrix metalloproteinases are an important group of zinc enzymes responsible for degradation of the extracellular matrix components such as collagen and proteoglycans in normal embryogenesis and remodeling and in many disease processes such as arthritis, cancer, periodontitis, and osteoporosis. A
S Netzel-Arnett et al.
Analytical biochemistry, 195(1), 86-92 (1991-05-15)
Four new fluorogenic heptapeptide substrates have been synthesized with sequences that are optimized for five human matrix metalloproteinases (MMP). All four substrates are similar to one recently reported by Stack and Gray (1989, J. Biol. Chem. 264, 4277-4281) and have
D E Kleiner et al.
Analytical biochemistry, 218(2), 325-329 (1994-05-01)
Zymography is an electrophoretic technique used to identify proteolytic activity in enzymes separated in polyacrylamide gels under nonreducing conditions. It has been used extensively in the qualitative evaluation of proteases present in tumors and cell culture conditioned media. Using commercially
L A Liotta et al.
Seminars in cancer biology, 1(2), 99-106 (1990-04-01)
The invasion and metastasis of cancer cells is a complex multistep process involving attachment of tumor cells to the basement membrane, proteolysis of the local connective tissue stroma, and migration through the proteolyzed stroma. Recent evidence implicates metalloproteinases such as
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique