8.52102
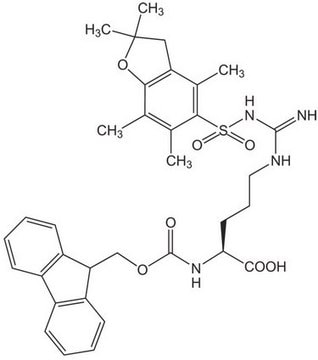
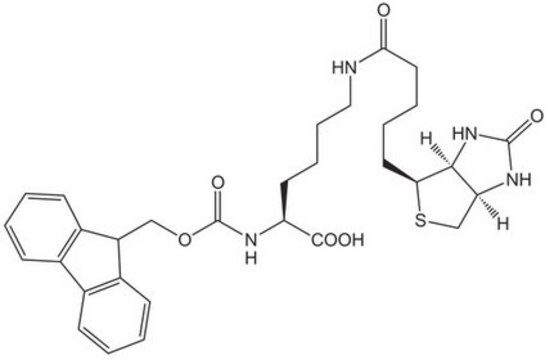
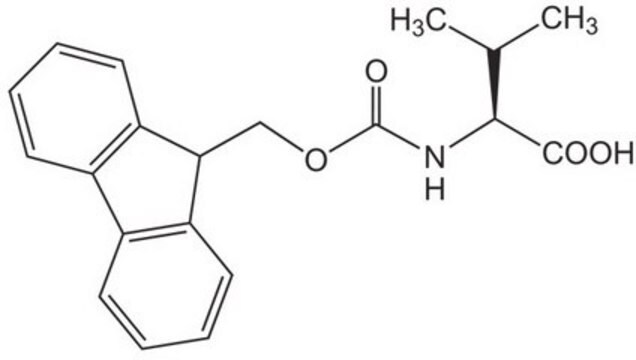
Fmoc-Glu(biotinyl-PEG)-OH
≥97% (TLC), for peptide synthesis, Novabiochem®
Synonyme(s) :
Fmoc-Glu(biotinyl-PEG)-OH, N-α-Fmoc-N-γ-(N-biotinyl-3-(2-(2-(3-aminopropyloxy)-ethoxy)-ethoxy)-propyl)-L-glutamine
About This Item
Produits recommandés
Nom du produit
Fmoc-Glu(biotinyl-PEG)-OH, Novabiochem®
Niveau de qualité
Gamme de produits
Novabiochem®
Essai
≥95.0% (HPLC)
≥97% (TLC)
Forme
powder
Capacité de réaction
reaction type: Fmoc solid-phase peptide synthesis
Fabricant/nom de marque
Novabiochem®
Application(s)
peptide synthesis
Groupe fonctionnel
biotin
Température de stockage
2-8°C
Chaîne SMILES
S1[C@H]([C@H]5NC(=O)N[C@H]5C1)CCCCC(=O)NCCCOCCOCCOCCCNC(=O)CC[C@H](NC(=O)OCC2c3c(cccc3)c4c2cccc4)C(=O)O
InChI
1S/C40H55N5O10S/c46-35(14-6-5-13-34-37-33(26-56-34)43-39(50)45-37)41-17-7-19-52-21-23-54-24-22-53-20-8-18-42-36(47)16-15-32(38(48)49)44-40(51)55-25-31-29-11-3-1-9-27(29)28-10-2-4-12-30(28)31/h1-4,9-12,31-34,37H,5-8,13-26H2,(H,41,46)(H,42,47)(H,44,51)(H,48,49)(H2,43,45,50)/t32-,33-,34-,37-/m0/s1
Clé InChI
MGOWNVYDCIBVKC-FNHRVDEZSA-N
Catégories apparentées
Description générale
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Biotinylation Reagents for Peptide Synthesis
Literature references
[1] B. Baumeister, et al. (2003) Biopolymers, 71, 339.
[2] X. Zhou et al., (2004) J. Am. Chem. Soc., 126, 15656.
[3] B. F. Gilmore, et al. (2006) Biochem. Biophys. Res. Commun,, 347, 373.
[4] C. T. Archer, et al. (2005) Mol. BioSyst., 1, 366.
Application
- Unbiased peptoid combinatorial cell screen identifies plectin protein as a potential biomarker for lung cancer stem cells: Utilized Fmoc-Glu(biotinyl-PEG)-OH in the synthesis protocol for peptoid libraries, contributing to biomarker discovery in lung cancer research (AC Raymond et al., 2019).
- Identification of side arm-modified DOTA scaffolds as multi-site binding ligands for cancer cells over normal cells: Included Fmoc-Glu(biotinyl-PEG)-OH in a synthesis protocol to enhance biotinylated scaffold properties for selective cancer cell targeting (V Rustagi, DG Udugamasooriya, 2019).
- TANGO-inspired design of anti-amyloid cyclic peptides: Employed Fmoc-Glu(biotinyl-PEG)-OH in the synthesis of cyclic peptides aimed at studying amyloid protein interactions, crucial for Alzheimer′s disease research (X Lu, RM Murphy, 2016).
- Converting a weaker ATP-binding site inhibitor into a potent hetero-bivalent ligand by tethering to a unique peptide sequence derived from the same kinase: Used Fmoc-Glu(biotinyl-PEG)-OH in developing new kinase inhibitors with improved binding properties (SR Kedika, DG Udugamasooriya, 2018).
Liaison
Remarque sur l'analyse
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (TLC(CMA2)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (0,2 mmol in 1 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Biotin-labelled peptides find applications in immunology and histochemistry for affinity purification and receptor localization.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique