800814P
Avanti
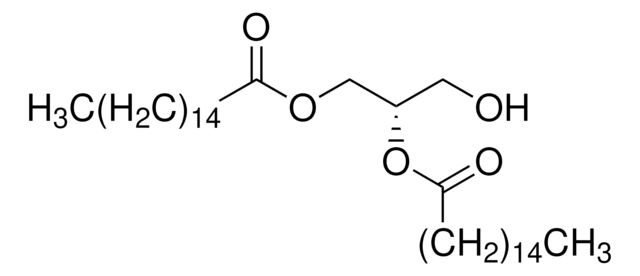
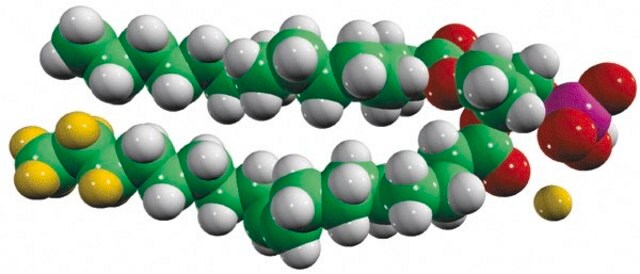
14:0 DG
1,2-dimyristoyl-sn-glycerol, powder
Synonyme(s) :
1,2-ditetradecanoyl-sn-glycerol; DG(14:0/14:0/0:0)
About This Item
Produits recommandés
Essai
>99% (TLC)
Forme
powder
Conditionnement
pkg of 1 × 10 mg (800814P-10mg)
pkg of 1 × 25 mg (800814P-25mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand 800814P
Type de lipide
neutral glycerides
neutral lipids
Conditions d'expédition
dry ice
Température de stockage
−20°C
InChI
1S/C31H60O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-30(33)35-28-29(27-32)36-31(34)26-24-22-20-18-16-14-12-10-8-6-4-2/h29,32H,3-28H2,1-2H3/t29-/m0/s1
Clé InChI
JFBCSFJKETUREV-LJAQVGFWSA-N
Description générale
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Application
- in the reconstitution of dry lipids for thin layer chromatography
- in lipid nanoparticles for RNA delivery studies
- as a standard in gas chromatography–mass spectrometry (GC-MS) analysis for the quantification of lipid A diacylglycerols
Actions biochimiques/physiologiques
Conditionnement
Stockage et stabilité
Autres remarques
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![23:2 Diyne PE [DC(8,9)PE] 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphoethanolamine, powder](/deepweb/assets/sigmaaldrich/product/images/228/422/4e95f75c-14fa-4117-a383-2eff73fa927f/640/4e95f75c-14fa-4117-a383-2eff73fa927f.jpg)