W266418
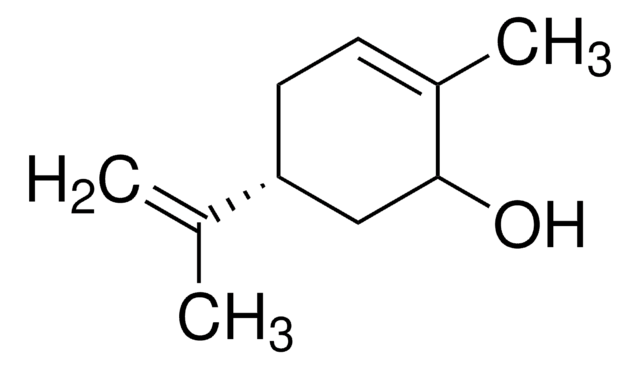
(S)-(−)-Perillyl alcohol
≥95%, FG
Synonyme(s) :
p-Mentha-1,8-diene-7-ol
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Qualité
FG
Halal
Kosher
Conformité réglementaire
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Essai
≥95%
Activité optique
[α]20/D −88°, c = 1 in methanol
Indice de réfraction
n20/D 1.501 (lit.)
pb
119-121 °C/11 mmHg (lit.)
Densité
0.96 g/mL at 25 °C (lit.)
Application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
Allergène alimentaire
no known allergens
Propriétés organoleptiques
fatty; green
Chaîne SMILES
CC(=C)[C@H]1CCC(CO)=CC1
InChI
1S/C10H16O/c1-8(2)10-5-3-9(7-11)4-6-10/h3,10-11H,1,4-7H2,2H3/t10-/m1/s1
Clé InChI
NDTYTMIUWGWIMO-SNVBAGLBSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- CYP108N12 initiates p-cymene biodegradation in Rhodococcus globerulus.: This study explores the enzymatic breakdown pathways of monoterpenes, using (S)-(−)-Perillyl alcohol as a precursor, offering insights into microbial degradation processes that could be vital for bioremediation efforts or synthetic biology applications (Giang et al., 2022).
- Orofacial antinociceptive effects of perillyl alcohol associated with codeine and its possible modes of action.: Research demonstrates the pain-relieving properties of (S)-(−)-Perillyl alcohol when combined with codeine, highlighting its potential for developing new analgesic formulations in dental and facial pain management (Limeira et al., 2022).
- Orofacial antinociceptive activity of (S)-(-)-perillyl alcohol in mice: a randomized, controlled and triple-blind study.: This study underpins the effectiveness of (S)-(−)-Perillyl alcohol in reducing orofacial pain in a controlled experimental setup, providing a basis for further clinical trials in pain management (Tomaz-Morais et al., 2017).
- In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol.: Highlights the anti-tumor properties of a novel derivative of (S)-(−)-Perillyl alcohol, suggesting its potential as a therapeutic agent in oncology, with comprehensive studies on its efficacy and safety (Andrade et al., 2016).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
230.0 °F - closed cup
Point d'éclair (°C)
110 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Global Trade Item Number
| Référence | GTIN |
|---|---|
| W266418-1KG | |
| W266418-1KG-K | 4061837800429 |
| W266418-5KG | |
| W266418-100G | |
| W266418-100G-K | 4061837800412 |
| W266418-5KG-K | 4061836712150 |
| W266418-SAMPLE | |
| W266418-SAMPLE-K | 4061837800436 |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique