P56100
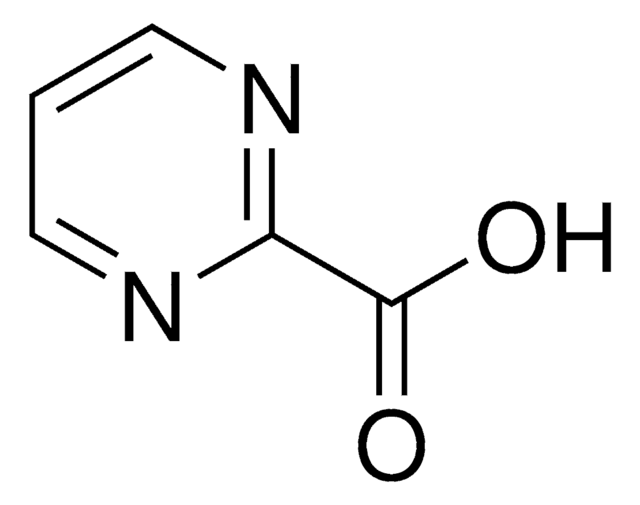
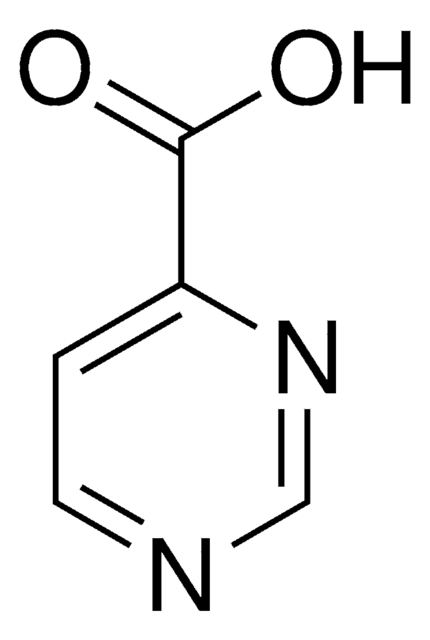
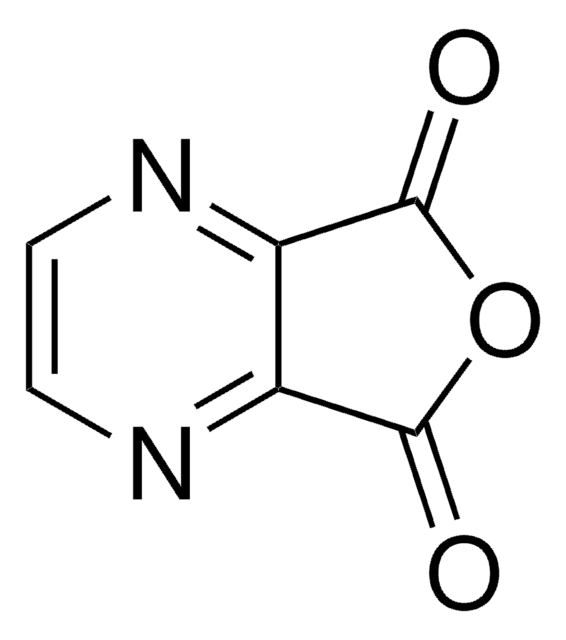
Pyrazinecarboxylic acid
99%
Synonyme(s) :
Pyrazinoic acid
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C5H4N2O2
Numéro CAS:
Poids moléculaire :
124.10
Numéro Beilstein :
112305
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
99%
Forme
powder
Pf
222-225 °C (dec.) (lit.)
Chaîne SMILES
OC(=O)c1cnccn1
InChI
1S/C5H4N2O2/c8-5(9)4-3-6-1-2-7-4/h1-3H,(H,8,9)
Clé InChI
NIPZZXUFJPQHNH-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Mohamed Abdel-Aziz et al.
European journal of medicinal chemistry, 45(8), 3384-3388 (2010-05-22)
A series of pyrazine-2-carboxylic acid hydrazide derivatives were synthesized and screened for their activity against Mycobacterium tuberculosis. The results show that pyrazine-2-carboxylic acid hydrazide-hydrazone derivatives 3a-l were less active than pyrazinamide. In contrast, the N(4)-ethyl-N(1)-pyrazinoyl-thiosemicarbazide 4 showed the highest activity
Rustam Z Khaliullin et al.
The journal of physical chemistry. B, 109(38), 17984-17992 (2006-07-21)
Experimental studies by Shul'pin and co-workers have shown that vanadate anions in combination with pyrazine-2-carboxylic acid (PCA identical with pcaH) produce an exceptionally active complex that promotes the oxidation of alkanes and other organic molecules. Reaction of this complex with
Wolfgang Holzer et al.
Magnetic resonance in chemistry : MRC, 47(7), 617-624 (2009-04-30)
NMR spectroscopic studies are undertaken with derivatives of 2-pyrazinecarboxylic acid. Complete and unambiguous assignment of chemical shifts ((1)H, (13)C, (15)N) and coupling constants ((1)H,(1)H; (13)C,(1)H; (15)N,(1)H) is achieved by combined application of various 1D and 2D NMR spectroscopic techniques. Unequivocal
Ping Lu et al.
Antimicrobial agents and chemotherapy, 55(11), 5354-5357 (2011-08-31)
Pyrazinoic acid, the active form of the first-line antituberculosis drug pyrazinamide, decreased the proton motive force and respiratory ATP synthesis rates in subcellular mycobacterial membrane assays. Pyrazinoic acid also significantly lowered cellular ATP levels in Mycobacterium bovis BCG. These results
Mirko Zimic et al.
Tuberculosis (Edinburgh, Scotland), 92(1), 84-91 (2011-10-19)
Pyrazinamide is one of the most important drugs in the treatment of latent Mycobacterium tuberculosis infection. The emergence of strains resistant to pyrazinamide represents an important public health problem, as both first- and second-line treatment regimens include pyrazinamide. The accepted
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique