930431
SuTEx1-alkyne
≥95%
Synonyme(s) :
N-2-propyn-1-yl-4-(1H-1,2,4-triazol-1-ylsulfonyl)-benzamide
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Description
Application: Chemoproteomics
Niveau de qualité
Pureté
≥95%
Forme
powder
Pf
155 °C
Température de stockage
−20°C
Chaîne SMILES
O=C(C1=CC=C(S(N2N=C([H])N=C2)(=O)=O)C=C1)NCC#C
Application
SuTEx1-alkyne is a probe that uses sulfur-triazole exchange chemistry to label tyrosines. A method was developed using cysteine-reactive compounds including this one to allow for unbiased analysis of proteomic data in quantitative applications (Zanon et al. 2021). The method uses light or heavy labelling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis (Zanon et al. 2020). Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow (Weerapana et al. 2010, Backus et al. 2016)
Autres remarques
Profiling the proteome-wide selectivity of diverse electrophiles
A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
Produit(s) apparenté(s)
Réf. du produit
Description
Tarif
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Ping Yu et al.
Nucleosides, nucleotides & nucleic acids, 40(7), 754-766 (2021-06-29)
We report herein comprehensive investigations of alkylation/sulfur exchange reactions of sulfur-containing substrates including nucleosides such as s2U, m5s2U, s4U, s2A and s2T-incorporated DNA enable by comprehensive screenings of the reagents (2a-2h). It has been proven that iodoacetamide (2a) displays the
Rui Sun et al.
Chemical research in toxicology, 30(10), 1797-1803 (2017-09-30)
Reactive metabolites (RM) formed from bioactivation of drugs can covalently modify liver proteins and cause mechanism-based inactivation of major cytochrome P450 (CYP450) enzymes. Risk of bioactivation of a test compound is routinely examined as part of lead optimization efforts in
Yide He et al.
Talanta, 134, 468-475 (2015-01-27)
In this work, we present a two-step labeling approach for the efficient tagging with lanthanide-containing complexes. For this purpose, derivatization of the cysteine residues with an alkyne group acting as linker was done before the DOTA complex was introduced using
Zarko V Boskovic et al.
ACS chemical biology, 11(7), 1844-1851 (2016-04-12)
Unbiased binding assays involving small-molecule microarrays were used to identify compounds that display unique patterns of selectivity among members of the zinc-dependent histone deacetylase family of enzymes. A novel, hydroxyquinoline-containing compound, BRD4354, was shown to preferentially inhibit activity of HDAC5
Yide He et al.
Journal of proteomics, 136, 68-76 (2015-12-30)
In a proof of concept study, metal-coded affinity tags based on click chemistry (MeCAT-Click) were used to analyze the proteome of Escherichia coli (E. coli) in response to heat stress. This allows high labeling efficiency, high detection sensitivity, and multiplex
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique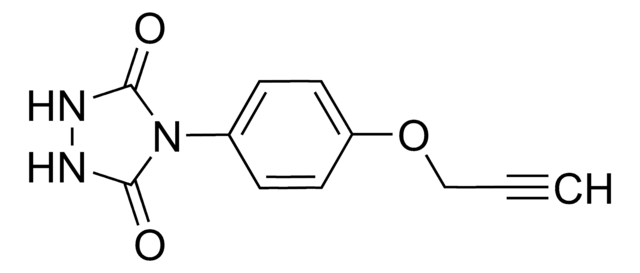
![Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)