900400
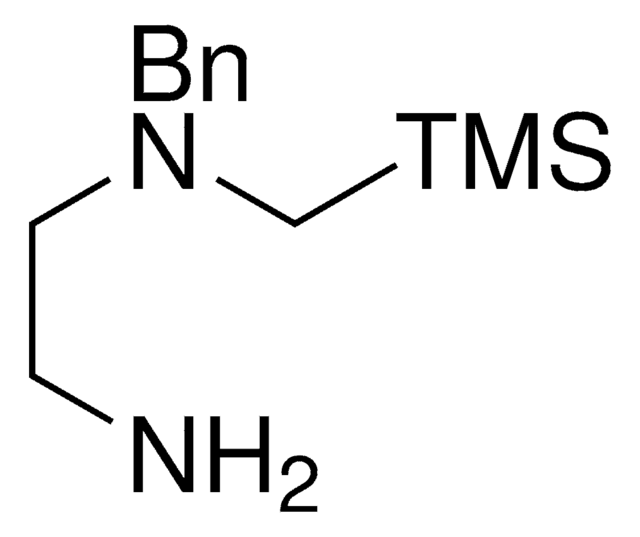
SLAP 3-SpiroCyHex N-Bn Pip Reagent
≥95%
Synonyme(s) :
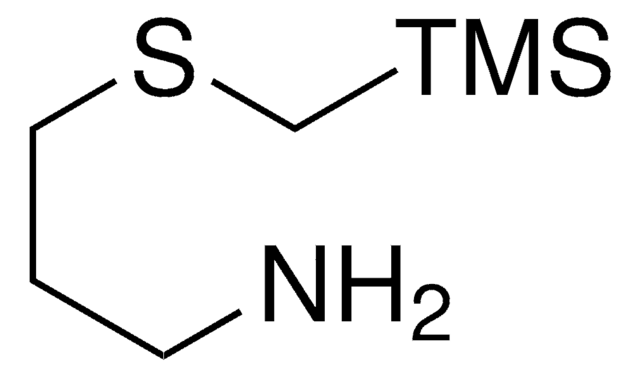
1-((Benzyl((trimethylsilyl)methyl)amino)methyl)cyclohexanamine
About This Item
Produits recommandés
Essai
≥95%
Forme
liquid
Indice de réfraction
n/D 1.516
Densité
0.956 g/mL
Groupe fonctionnel
amine
phenyl
Température de stockage
−20°C
InChI
1S/C18H32N2Si/c1-21(2,3)16-20(14-17-10-6-4-7-11-17)15-18(19)12-8-5-9-13-18/h4,6-7,10-11H,5,8-9,12-16,19H2,1-3H3
Clé InChI
HHDPYUUHZQUCNL-UHFFFAOYSA-N
Application
Autres remarques
- Technology Spotlight: SLAP Reagents for Piperazine Synthesis
- Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones
- Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents.
- Continuous Flow Synthesis of Morpholines and Oxazepanes with Silicon Amine Protocol (SLAP) Reagents and Lewis Acid Facilitated Photoredox Catalysis
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Protocoles
The Bode group has developed SnAP (stannyl amine protocol) reagents that cross-couple with aldehydes and ketones to provide one-step access to a wide variety of saturated N-heterocycles.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique