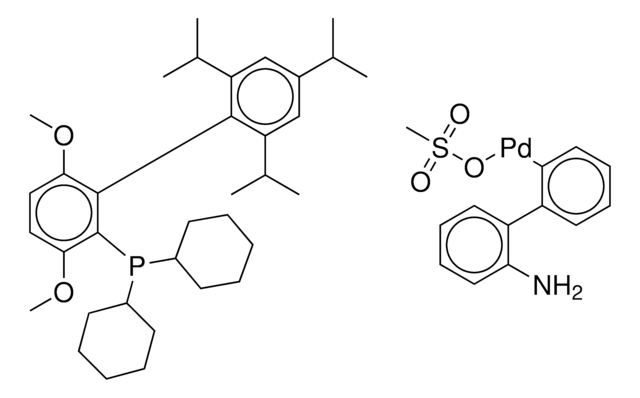
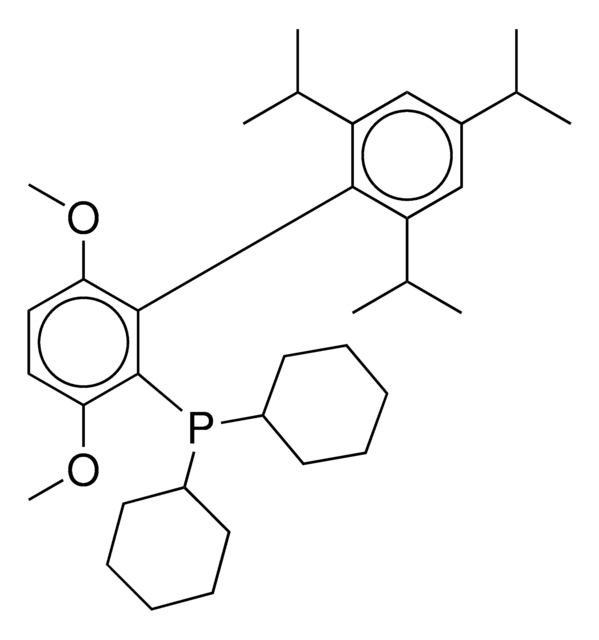
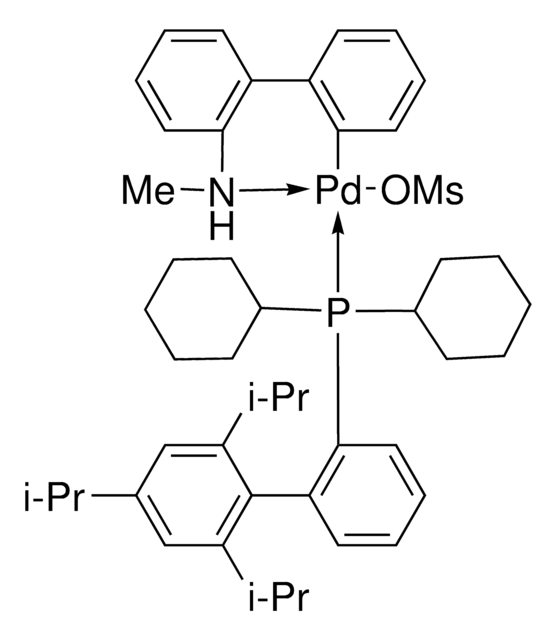
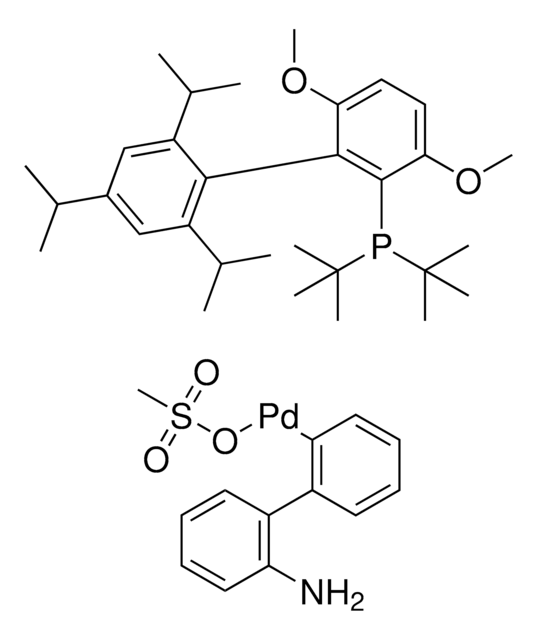
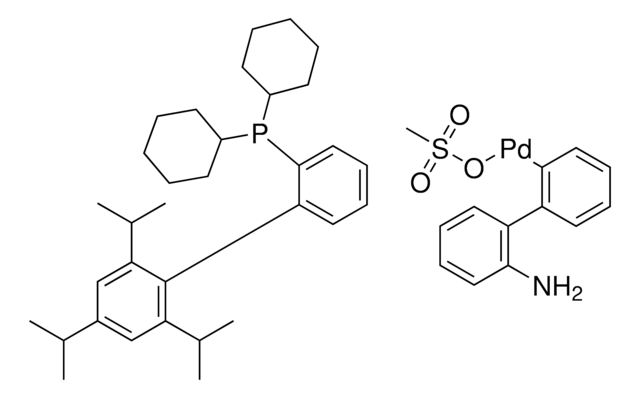
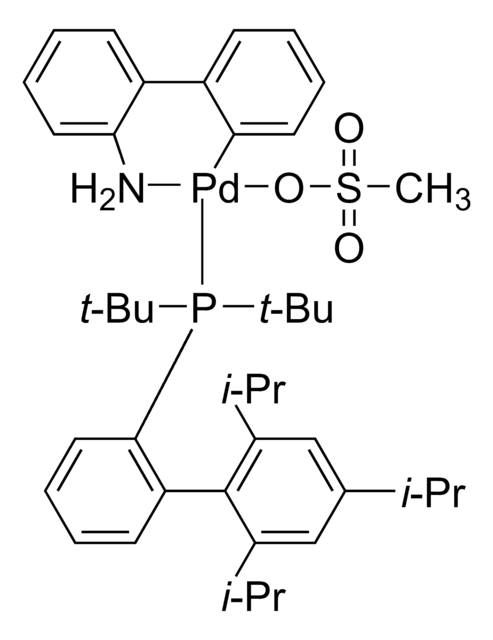
804355
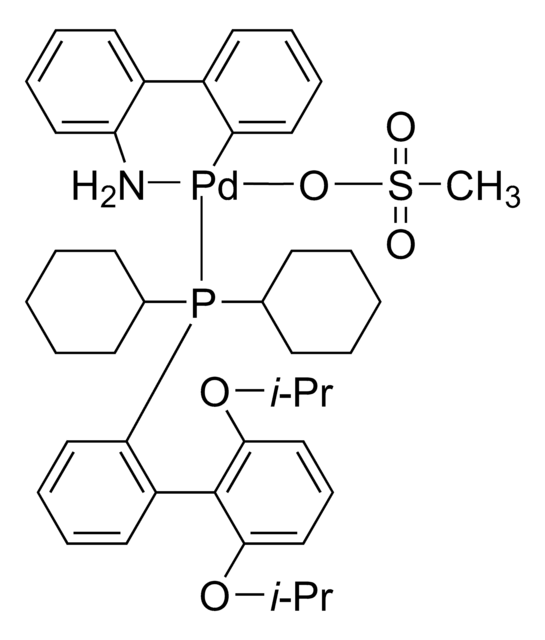
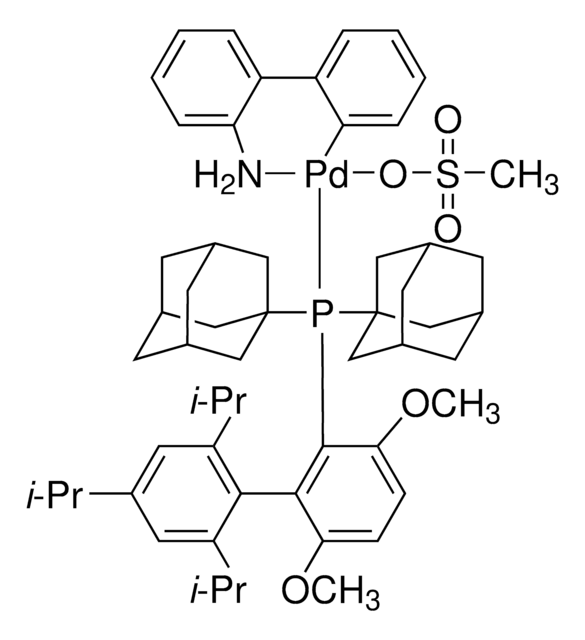
BrettPhos Pd G4
Synonyme(s) :
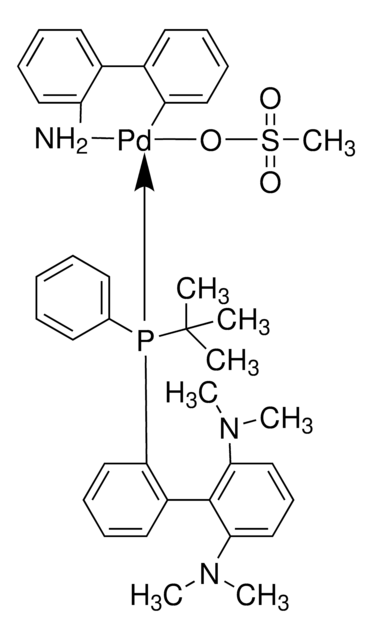
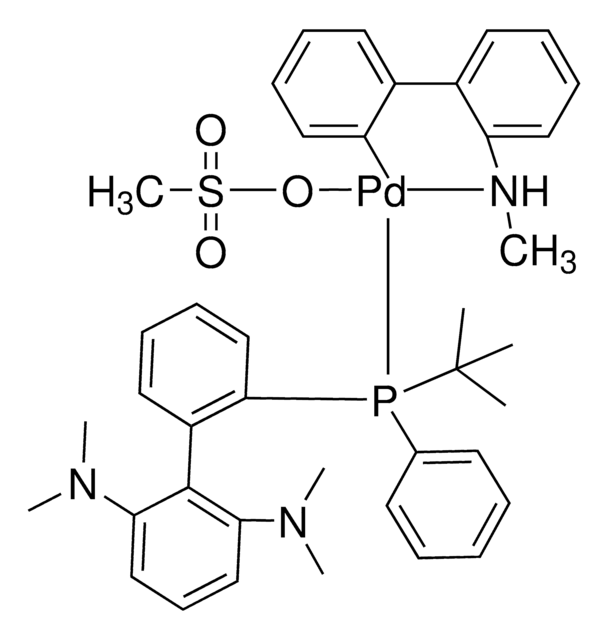
(SP-4-3)-[Dicyclohexyl[3,6-dimethoxy-2′,4′,6′-tris(1-methylethyl)[1,1′-biphenyl]-2-yl]phosphine-κP](methanesulfonato-κO)[2′-(methylamino-κN)[1,1′-biphenyl]-2-yl-κC]
About This Item
Produits recommandés
Pureté
95%
Niveau de qualité
Forme
powder
Caractéristiques
generation 4
Capacité de réaction
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
Pertinence de la réaction
reagent type: catalyst
reaction type: Cross Couplings
Groupe fonctionnel
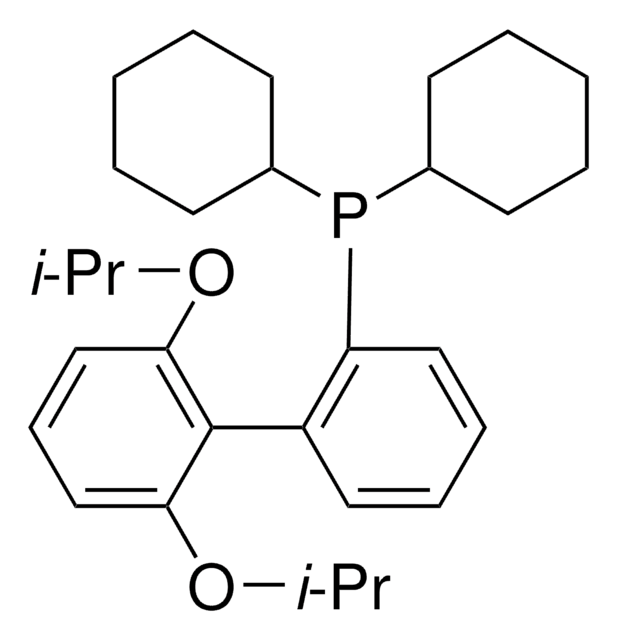
phosphine
Chaîne SMILES
CC(C)C1=CC(C(C)C)=CC(C(C)C)=C1C2=C(P(C3CCCCC3)C4CCCCC4)C(OC)=CC=C2OC.CNC5=C(C6=C([Pd]OS(C)(=O)=O)C=CC=C6)C=CC=C5
InChI
1S/C35H53O2P.C13H12N.CH4O3S.Pd/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28;1-14-13-10-6-5-9-12(13)11-7-3-2-4-8-11;1-5(2,3)4;/h19-25,27-28H,9-18H2,1-8H3;2-7,9-10,14H,1H3;1H3,(H,2,3,4);/q;;;+1/p-1
Clé InChI
YVIWYUQZBYFFLZ-UHFFFAOYSA-M
Description générale
Application
- High Throughput Experimentation in Pharmaceutical Process Chemistry: This study evaluates the efficacy of BrettPhos Pd G4 in a ring closure reaction, emphasizing its selectivity and yield, which supports its potential utility in pharmaceutical synthesis (Solazzo, 2022).
- Synthesis and characterization of fluorescent amino acid dimethylaminoacridonylalanine: Research detailing the use of various Buchwald precatalysts including BrettPhos Pd G4. The study explores the synthesis of a fluorescent amino acid, highlighting the specific conditions where BrettPhos Pd G4 provided notable benefits over other catalysts (Jones et al., 2021).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Contenu apparenté
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique