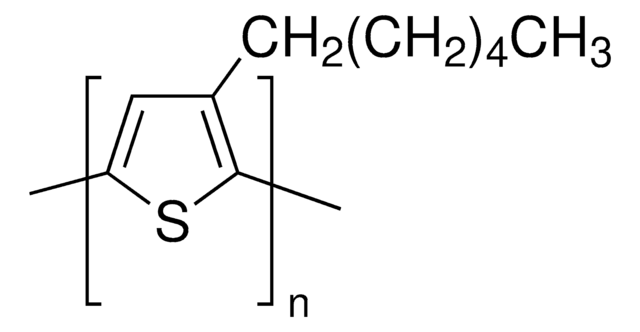
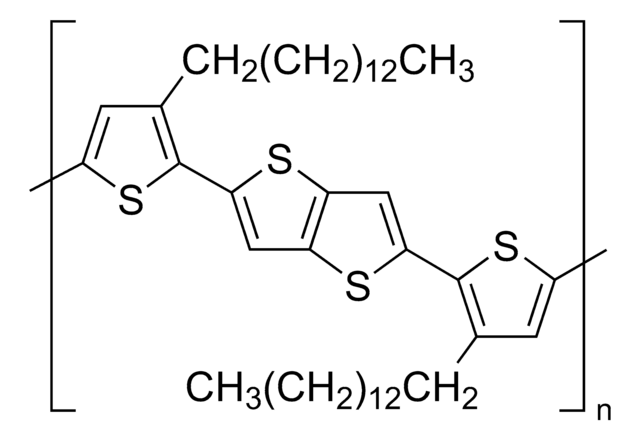
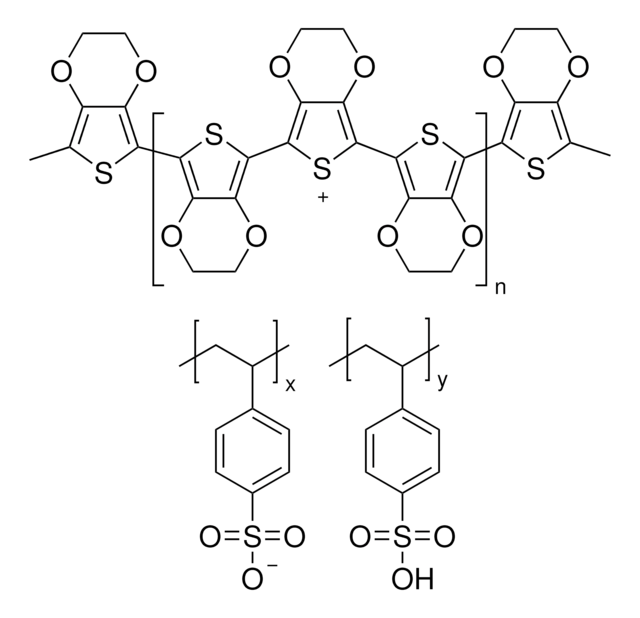
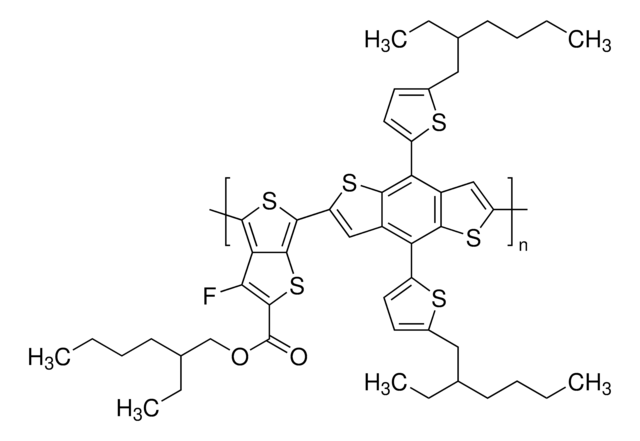
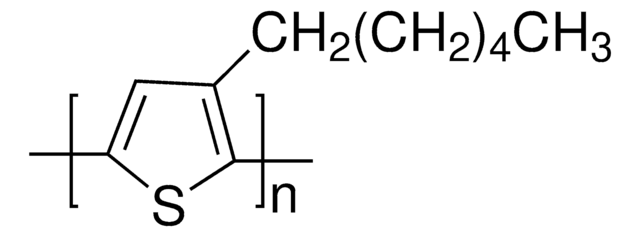
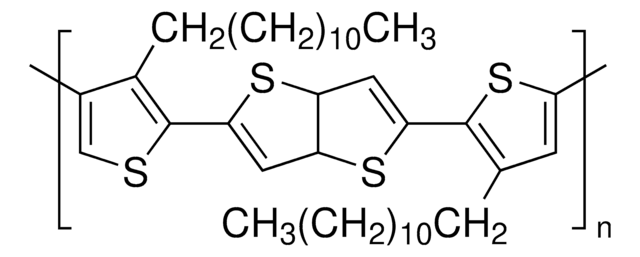
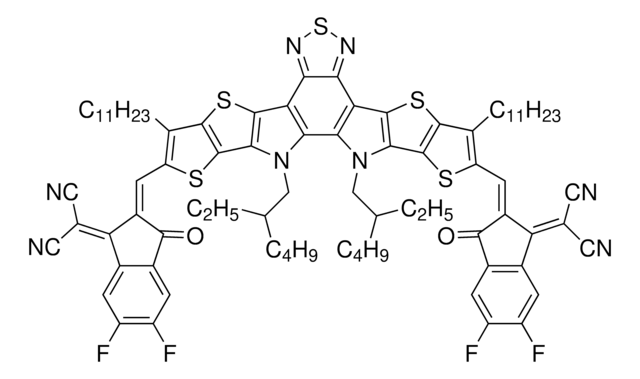
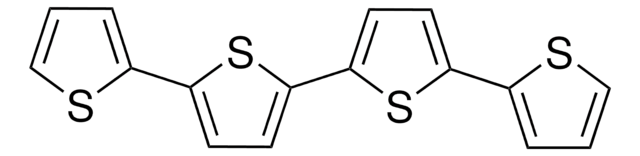
764639
5,5′′′-Bis(tridecafluorohexyl)-2,2′:5′,2 ′′:5′′,2′′′-quaterthiophene
Synonyme(s) :
α,ω-Diperfluorohexyl-quarterthiophene, DFH-4T
About This Item
Produits recommandés
Forme
solid
Pf
205-210 °C
Propriétés du semi-conducteur
N-type (mobility≤0.64 cm2/V·s)
Chaîne SMILES
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)c1ccc(s1)-c2ccc(s2)-c3ccc(s3)-c4ccc(s4)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C28H8F26S4/c29-17(30,19(33,34)21(37,38)23(41,42)25(45,46)27(49,50)51)15-7-5-13(57-15)11-3-1-9(55-11)10-2-4-12(56-10)14-6-8-16(58-14)18(31,32)20(35,36)22(39,40)24(43,44)26(47,48)28(52,53)54/h1-8H
Clé InChI
UBMTYFFPSPVBSP-UHFFFAOYSA-N
Description générale
Application
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
There is widespread demand for thin, lightweight, and flexible electronic devices such as displays, sensors, actuators, and radio-frequency identification tags (RFIDs). Flexibility is necessary for scalability, portability, and mechanical robustness.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique