743062
TurboBeads™ Complexon
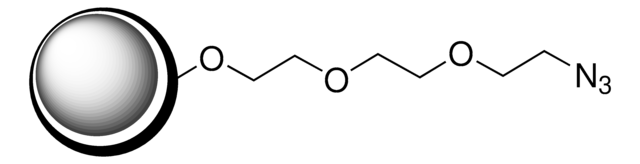
extent of labeling: ≥0.1 mmol/g loading (-Ph-CH2-N(CH2-COOH)2)
Synonyme(s) :
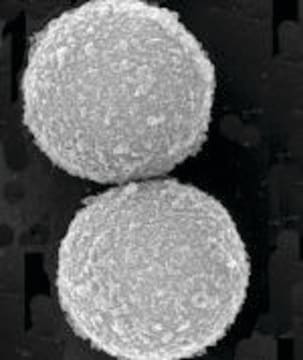
Nano particles, magnetic, IDA functionalized
About This Item
Produits recommandés
Gamme de produits
TurboBeads™
Pureté
≥99%
Forme
powder
Composition
carbon content, ≤14 wt. %
Capacité de réaction
reaction type: solution phase peptide synthesis
Pertinence de la réaction
reactivity: copper reactive
Ampleur du marquage
≥0.1 mmol/g loading (-Ph-CH2-N(CH2-COOH)2)
Magnétisation
≥120 emu/g, mass saturation
Superficie
≥15 m2/g
Diamètre moyen
≤50 nm
Adéquation
conforms to structure for Infrared spectrum
Conditionnement
Remarque sur l'analyse
air-stability:
weight gain in air at 400°C >20 wt.%
weight gain in air at 100°C <3 wt.%
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique