688738
Basolite® A100 TAB 3x3
produced by BASF
Synonyme(s) :
Aluminum terephthalate, MIL 53
About This Item
Produits recommandés
Qualité
produced by BASF
Forme
tablet
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Superficie
1100-1500 m2/g
Répartition Des Tailles De Particules
31.55 μm (D50)
Masse volumique apparente
0.4 g/cm3
Autre catégorie plus écologique
, Enabling
Description générale
Application
Autres remarques
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Repr. 1B
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
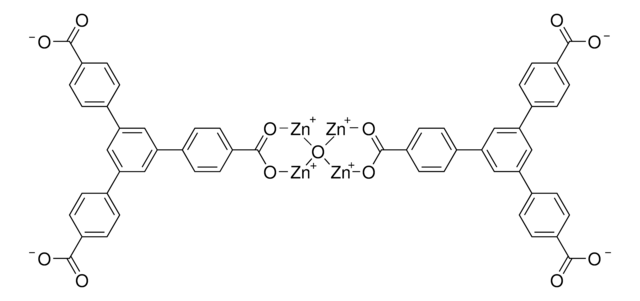
We are pleased to offer MOFs under the tradename Basolite.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![Zinc bis[bis(trimethylsilyl)amide] 97%](/deepweb/assets/sigmaaldrich/product/structures/294/819/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d/640/cd22dd81-f7c8-4f0c-944e-1b74c1ad5e6d.png)
![(11bR, 11′bR)-4,4′-9,9-Dimethyl-9H-xanthene-4,5-diyl)bis-dinaphtho[2,1-d:1′, 2′-f][1,3,2]dioxaphosphepin](/deepweb/assets/sigmaaldrich/product/structures/198/331/bd88130d-f49e-4bc8-b82e-5e43b3bcea95/640/bd88130d-f49e-4bc8-b82e-5e43b3bcea95.png)