672068
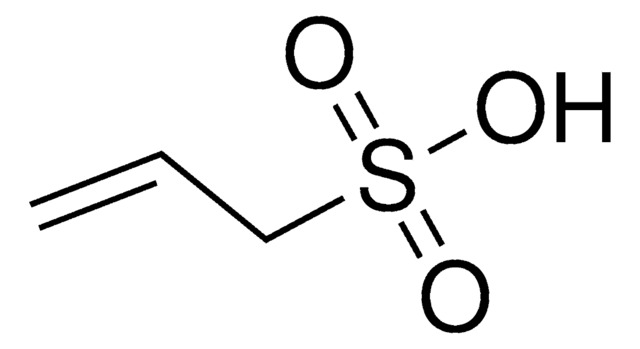
Vinylphosphonic acid
≥90% (T)
Synonyme(s) :
Ethenephosphonic acid, Ethylenephosphonic acid, P-Ethenylphosphonic acid
About This Item
Produits recommandés
Pureté
≥90% (T)
Impuretés
≤7.0% water
Pf
36 °C (Lit. dry VPA) (lit.)
Densité
1.37 g/mL at 20 °C (lit.)
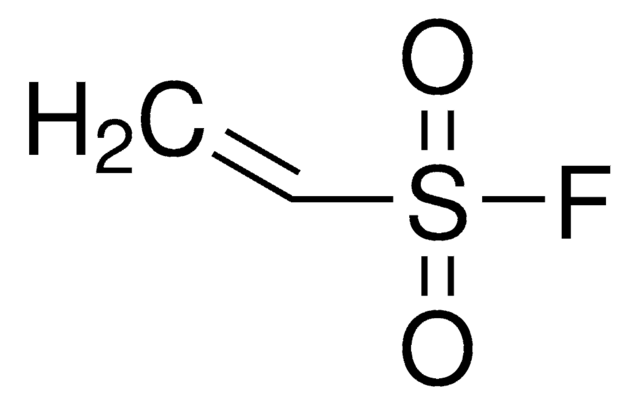
Chaîne SMILES
OP(O)(=O)C=C
InChI
1S/C2H5O3P/c1-2-6(3,4)5/h2H,1H2,(H2,3,4,5)
Clé InChI
ZTWTYVWXUKTLCP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Met. Corr. 1 - Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
467.6 °F
Point d'éclair (°C)
242 °C
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)