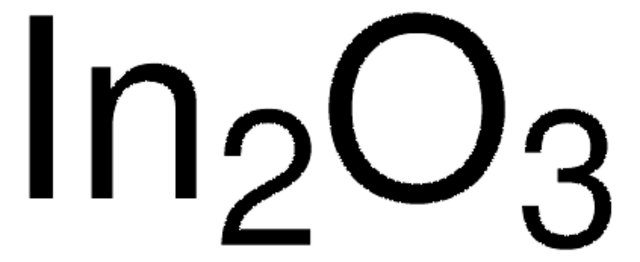483702
Germanium(IV) oxide
≥99.99% trace metals basis
Synonyme(s) :
Germanium dioxide
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥99.99% trace metals basis
Forme
powder
Pf
>400 °C (lit.)
Solubilité
ethylene glycol: soluble
Densité
4.23 g/cm3
Application(s)
battery manufacturing
Chaîne SMILES
O=[Ge]=O
InChI
1S/GeO2/c2-1-3
Clé InChI
YBMRDBCBODYGJE-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
White solid-state light can be generated using three different approaches: By employing three diodes that emit red, green and blue light respectively, by using a near-UV LED that excites several phosphors that emit over the complete spectral range, or the third, most widely used alternative entailing down-conversion of a portion of blue LED light to longer wavelengths in such a manner that white light emerges.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique