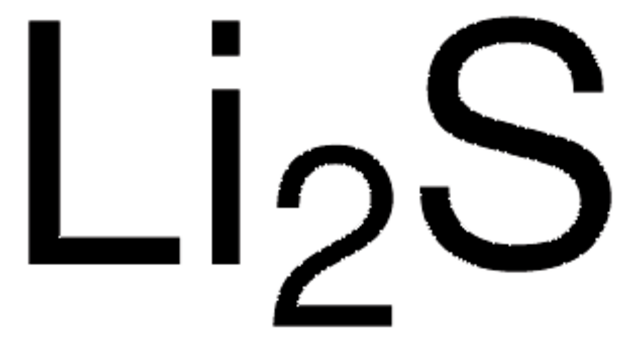439746
Lithium iodide
AnhydroBeads™, −10 mesh, 99.99% trace metals basis
Synonyme(s) :
Lithium monoiodide
About This Item
Produits recommandés
Gamme de produits
AnhydroBeads™
Niveau de qualité
Essai
99.99% trace metals basis
Forme
beads
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
≤150.0 ppm Trace Metal Analysis
Taille des particules
−10 mesh
Pf
446 °C (lit.)
Densité
3.49 g/mL at 25 °C (lit.)
Autre catégorie plus écologique
Chaîne SMILES
[Li+].[I-]
InChI
1S/HI.Li/h1H;/q;+1/p-1
Clé InChI
HSZCZNFXUDYRKD-UHFFFAOYSA-M
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- As a precursor to synthesize polymer-based electrolytes for dye-sensitized solar cell(DSSC) application via solution casting method.
- Li2S-P2S5-LiI crystalline inorganic-organic hybrid electrolytes with high ionic conductivity via liquid-phase synthesis for all solid-state batteries.
- As a redox mediator for Lithium–oxygen (Li–O2) batteries. It can facilitate redox reactions by shuttling charge carriers between electrodes, enabling efficient energy conversion.
Caractéristiques et avantages
- Excellent ionic conductivity at elevated temperature
- Good thermal stability
- Compatible with lithium-based battery materials.
Informations légales
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
À utiliser avec
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique