419060
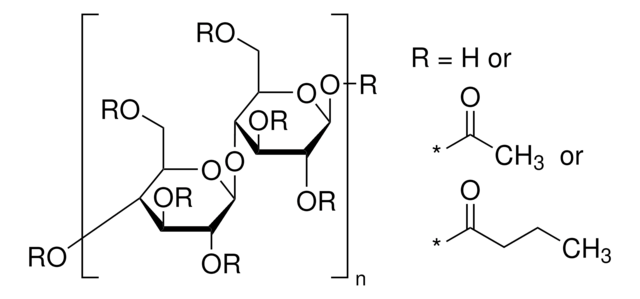
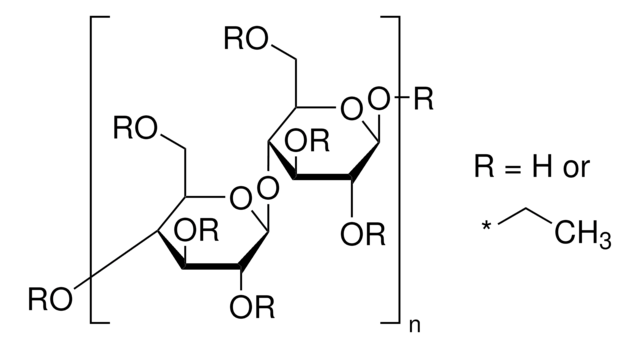
Cellulose acetate butyrate
average Mn ~30,000
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Forme
powder
Niveau de qualité
Poids mol.
average Mn ~30,000
Ampleur du marquage
≥49 wt. % Butyryl
1.4-2.4 wt. % Hydroxyl
Indice de réfraction
n20/D 1.475 (lit.)
Densité
1.25 g/mL at 25 °C (lit.)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
nwg
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Yasunori Miyazaki et al.
International journal of pharmaceutics, 258(1-2), 21-29 (2003-05-20)
The objective of this study was to evaluate mucoadhesive properties and gastrointestinal transit of microspheres made of oppositely charged dextran derivatives and cellulose acetate butyrate (CAB). The microspheres were prepared by emulsion solvent evaporation method. A reference microsphere was made
Min Soo Park et al.
Langmuir : the ACS journal of surfaces and colloids, 22(10), 4594-4598 (2006-05-03)
We investigate the effects of interfacial energy between water and solvent as well as polymer concentration on the formation of porous structures of polymer films prepared by spin coating of cellulose acetate butyrate (CAB) in mixed solvent of tetrahydrofuran (THF)
R B Umamaheshwari et al.
Drug delivery, 10(3), 151-160 (2003-08-29)
We prepared cellulose acetate butyrate (CAB)-coated cholestyramine microcapsules as a intragastric floating drug delivery system endowed with floating ability due to the carbon dioxide generation when exposed to the gastric fluid. The microcapsules also have a mucoadhesive property. Ion-exchange resin
Decheng Ma et al.
Journal of pharmaceutical and biomedical analysis, 35(4), 779-788 (2004-06-15)
The purpose of this study was to qualitatively and quantitatively determine potential cellulose acetate butyrate (CAB) extractables in a way to meaningfully predict the in vivo exposure resulting from clinical administration. Extractions of CAB-381-20 were performed in several solvent systems
W M Obeidat et al.
Journal of microencapsulation, 21(1), 47-57 (2004-01-14)
Theophylline microspheres were prepared by the emulsion-solvent evaporation method using cellulose acetate butyrate (CAB381-20) and mixtures of CAB381-20(R) and cellulose acetate phthalate. The physical state of the drug, polymers and microspheres surfaces were determined using scanning electron microscopy. For those
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique