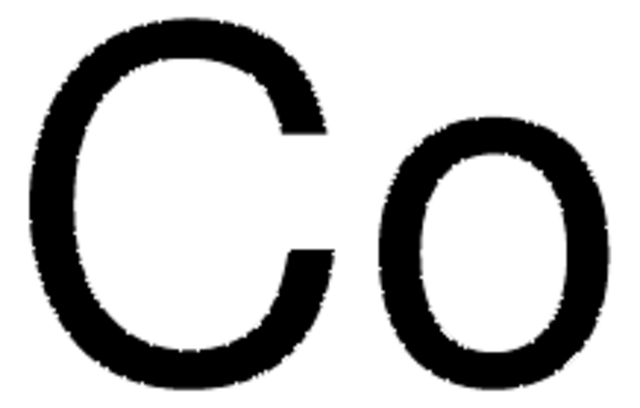356891
Cobalt
foil, thickness 1.0 mm, 99.95% trace metals basis
Synonyme(s) :
Cobalt element, Cobalt-59
About This Item
Produits recommandés
Niveau de qualité
Pureté
99.95% trace metals basis
Forme
foil
Résistivité
6.24 μΩ-cm, 20°C
Épaisseur
1.0 mm
Point d'ébullition
2900 °C (lit.)
Densité
8.9 g/mL at 25 °C (lit.)
Chaîne SMILES
[Co]
InChI
1S/Co
Clé InChI
GUTLYIVDDKVIGB-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Quantité
50 × 50 mm (approximately 22.4 g)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 1B - Eye Irrit. 2 - Muta. 2 - Repr. 1A - Resp. Sens. 1 - Skin Sens. 1
Code de la classe de stockage
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Biomedical implants are essentially foreign substances within the human body that must survive many years’ exposure to demanding mechanical and physiological conditions. Despite these challenges, metal implants have been widely used to substitute for or rebuild hard tissues such as bones and teeth.
The unique properties of the rare-earth elements and their alloys have brought them from relative obscurity to high profile use in common hightech applications.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique