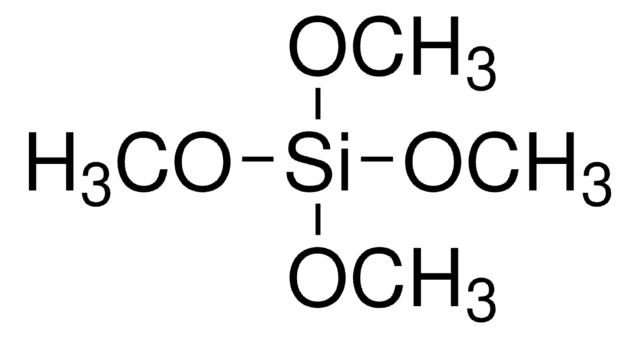341436
Tetramethyl orthosilicate
≥99%
Synonyme(s) :
Tetramethoxysilane
About This Item
Produits recommandés
Densité de vapeur
5.25 (vs air)
Niveau de qualité
Pression de vapeur
13 hPa ( 20 °C)
Pureté
≥99%
Forme
liquid
Indice de réfraction
n20/D 1.368 (lit.)
Point d'ébullition
121-122 °C (lit.)
Pf
−4 °C (lit.)
Densité
1.023 g/mL at 25 °C (lit.)
Chaîne SMILES
CO[Si](OC)(OC)OC
InChI
1S/C4H12O4Si/c1-5-9(6-2,7-3)8-4/h1-4H3
Clé InChI
LFQCEHFDDXELDD-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- As a precursor to synthesize organic-inorganic coating materials by sol-gel processing method.
- As an efficient reagent for direct amidation of aliphatic and aromatic carboxylic acids with amines and anilines.
- As a precursor to fabricate SiO2 nanocomposite films by chemical vapor deposition(CVD) method.
- As a selective catalyst ofC3-methylation of indole.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 1 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
78.8 °F - closed cup
Point d'éclair (°C)
26 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Research involving reactive silicone chemistry has focused on the production of pure silicon and hybrid materials, hydrosilylation, ring-opening and atom transfer polymerizations, polymerizations with controlled stereochemistry, and condensation reactions.
Silica is a very popular inorganic nanomaterial used in a wide range of applications including fillers for rubber, catalyst supports, separation media, carriers in food and agriculture, and abrasive/anticaking agents in cosmetics. It is also widely believed to be an important material for biomedical applications for following reasons.
Magnetism and magnetic materials have been of scientific interest for over 1,000 years. More recently, fundamental investigations have focused on exploring the various types of magnetic materials and understanding the magnetic effects created by electric currents.
Hybrid organic-inorganic sol-gel materials containing silica were first called “ORMOSILs” in 1984.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique