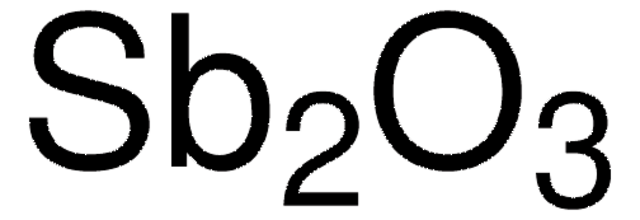230898
Antimony(III) oxide
powder, 5 μm, ReagentPlus®, 99%
Synonyme(s) :
Diantimony trioxide
About This Item
Produits recommandés
Gamme de produits
ReagentPlus®
Essai
99%
Forme
powder
Taille des particules
5 μm
pb
1550 °C (lit.)
Pf
655 °C (lit.)
Chaîne SMILES
O=[Sb]O[Sb]=O
InChI
1S/3O.2Sb
Clé InChI
ADCOVFLJGNWWNZ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Thermal Decomposition and Carbothermal Reduction: Examines the recycling and purification processes of Antimony(III) oxide from metal oxides, valuable for those interested in sustainable metallurgy (Karlsson et al., 2018).
- Oxidation of Antimony(III) in Soil by Manganese(IV) Oxide: Studies the interaction between manganese oxide and antimony(III) oxide in soils, providing insights into environmental chemistry and soil remediation (Fu et al., 2018).
- Adsorption of Antimony(V) onto Mn(II)-Enriched Surfaces: Focuses on how manganese oxide interacts with antimony(V), relevant for those studying adsorption processes and water treatment technologies (Liu et al., 2015).
- Kinetic Modeling of Antimony(III) Oxidation and Sorption in Soils: Provides a kinetic model of antimony(III) interactions with environmental matrices, useful for environmental scientists and chemists involved in pollution control (Cai et al., 2016).
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 2
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique