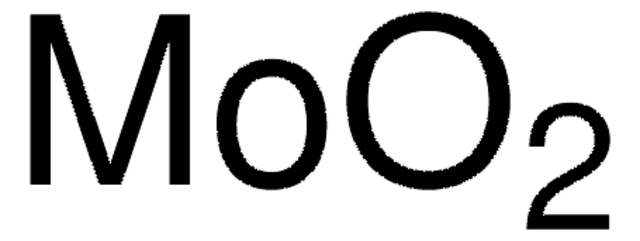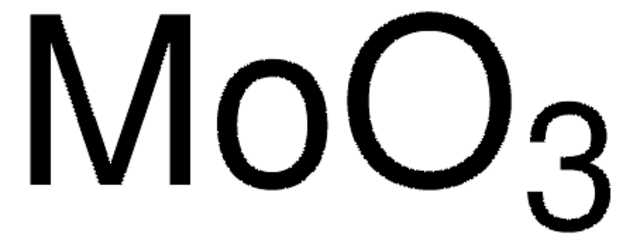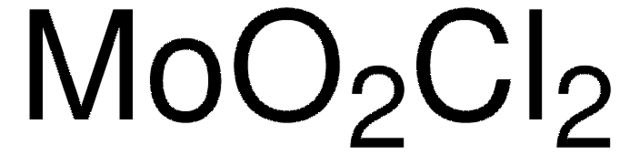203815
Molybdenum(VI) oxide
99.97% trace metals basis
Synonyme(s) :
Molybdenum trioxide
About This Item
Produits recommandés
Pureté
99.97% trace metals basis
Forme
powder
Pf
795 °C (lit.)
Application(s)
battery manufacturing
Chaîne SMILES
O=[Mo](=O)=O
InChI
1S/Mo.3O
Clé InChI
JKQOBWVOAYFWKG-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 2 - Eye Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure.
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
The production of hydrogen by catalytic water splitting is important for a wide range of industries including renewable energy petroleum refining and for the production of methanol and ammonia in the chemical industry.
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique