183172
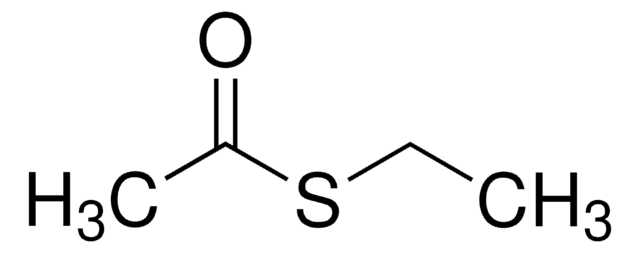
S-Phenyl thioacetate
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
CH3COSC6H5
Numéro CAS:
Poids moléculaire :
152.21
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
98%
Forme
liquid
Indice de réfraction
n20/D 1.57 (lit.)
pb
99-100 °C/6 mmHg (lit.)
Densité
1.124 g/mL at 25 °C (lit.)
Groupe fonctionnel
thioester
Température de stockage
2-8°C
Chaîne SMILES
CC(=O)Sc1ccccc1
InChI
1S/C8H8OS/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
Clé InChI
WBISVCLTLBMTDS-UHFFFAOYSA-N
Catégories apparentées
Application
S-Phenyl thioacetate was used as a substrate to measure the esterase activity.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
174.2 °F - closed cup
Point d'éclair (°C)
79 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Yu Yuan et al.
Journal of the American Chemical Society, 131(15), 5432-5437 (2009-04-22)
Described herein is the chemical synthesis of the Cys(29)-Gly(77) glycopeptide domain (22) of erythropoietin. Our initial ligation strategy targeted a C --> N termini condensation between glycopeptide 3 and peptide 4. However, the reaction was hindered by the "unattainable" reactivity
K Lorentz et al.
Clinica chimica acta; international journal of clinical chemistry, 308(1-2), 69-78 (2001-06-20)
Arylesterase (EC 3.1.1.2) activity in serum was specifically measured using thiophenyl acetate in a mechanized assay at 37 degrees C with 4-bromophenylboronic acid as inhibitor of cholinesterase and hexacyanoferrate-III as indicator. The systematic development of a routine method, apparent limitations
Anita Bosak et al.
Molecules (Basel, Switzerland), 25(1) (2020-01-18)
Mammalian paraoxonase-1 hydrolyses a very broad spectrum of esters such as certain drugs and xenobiotics. The aim of this study was to determine whether carbamates influence the activity of recombinant PON1 (rePON1). Carbamates were selected having a variety of applications:
D I Draganov et al.
The Journal of biological chemistry, 275(43), 33435-33442 (2000-08-10)
The paraoxonase gene family contains at least three members: PON1, PON2, and PON3. The physiological roles of the corresponding gene products are still uncertain. Until recently, only the serum paraoxonase/arylesterase (PON1) had been purified and characterized. Here we report the
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique