103578
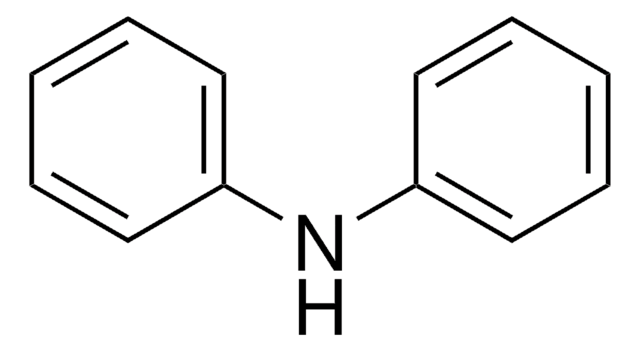
4-Nitrodiphenylamine
99%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
C6H5NHC6H4NO2
Numéro CAS:
Poids moléculaire :
214.22
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
eCl@ss :
39032032
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
99%
Forme
solid
Pf
132-135 °C (lit.)
Groupe fonctionnel
nitro
Chaîne SMILES
[O-][N+](=O)c1ccc(Nc2ccccc2)cc1
InChI
1S/C12H10N2O2/c15-14(16)12-8-6-11(7-9-12)13-10-4-2-1-3-5-10/h1-9,13H
Clé InChI
XXYMSQQCBUKFHE-UHFFFAOYSA-N
Catégories apparentées
Description générale
4-Nitrodiphenylamine undergoes heterogeneous catalytic transfer hydrogenation to form p-phenylenediamines. 4-Nitrodiphenylamine is used as stabilizer for propellants and explosives.
Application
4-Nitrodiphenylamine was used to study reduction of nitrated diphenylamine derivatives in sediment water batch enrichments and dense cell suspensions of anaerobic, aromatic-compound-mineralizing pure bacterial cultures.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Heterogeneous catalytic transfer hydrogenation of 4-nitrodiphenylamine to p-phenylenediamines.
Banerjee AA and Mukesh D.
Journal of the Chemical Society. Chemical Communications, 18, 1275-1276 (1988)
Anne-Laure Gassner et al.
Science & justice : journal of the Forensic Science Society, 60(2), 136-144 (2020-03-01)
The present study investigated the organic gunshot residue (OGSR) background level of police vehicles in Switzerland. Specimens from 64 vehicles belonging to two regional police services were collected and analysed by LC-MS in positive mode. The driver's and back seats
O Drzyzga et al.
Applied and environmental microbiology, 61(9), 3282-3287 (1995-09-01)
2-Nitrodiphenylamine, 4-nitrodiphenylamine, and 2,4-dinitrodiphenylamine were anaerobically metabolized in sediment-water batch enrichments inoculated with mud from the German North Sea coast. The first intermediate in 2,4-dinitrodiphenylamine degradation was 2-amino-4-nitrodiphenylamine, which appeared in large (nearly stoichiometric) amounts before being completely reduced to
Anne-Laure Gassner et al.
Science & justice : journal of the Forensic Science Society, 59(1), 58-66 (2019-01-19)
The present study aimed at providing data to assess the secondary transfer of organic gunshot residues (OGSR). Three scenarios were evaluated in controlled conditions, namely displacing a firearm from point A to point B, a simple handshake and an arrest
Zuriñe Abrego et al.
The Analyst, 139(23), 6232-6241 (2014-10-11)
A method based on scanning laser ablation and inductively coupled plasma-mass spectrometry (SLA-ICPMS) and Raman micro-spectroscopy for the detection and identification of compounds consistent with gunshot residue particles (GSR) has been developed. The method has been applied to the characterization
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique