102741
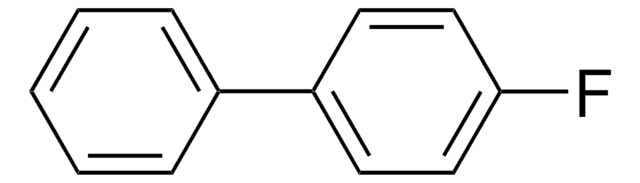
2-Fluorobiphenyl
96%
Synonyme(s) :
1-Fluoro-2-phenylbenzene, o-Fluorodiphenyl
About This Item
Produits recommandés
Niveau de qualité
Essai
96%
Forme
solid
pb
248 °C (lit.)
Pf
71-74 °C (lit.)
Groupe fonctionnel
fluoro
phenyl
Chaîne SMILES
Fc1ccccc1-c2ccccc2
InChI
1S/C12H9F/c13-12-9-5-4-8-11(12)10-6-2-1-3-7-10/h1-9H
Clé InChI
KLECYOQFQXJYBC-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Protocoles
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
US EPA Method 8270 (Appendix IX): GC Analysis of Semivolatiles on Equity®-5 (30 m x 0.25 mm I.D., 0.50 μm)
Global Trade Item Number
| Référence | GTIN |
|---|---|
| 102741-10G | 4061838344755 |
| 102741-1G | 4061838669995 |
| 102741-2.5G |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique