M0285
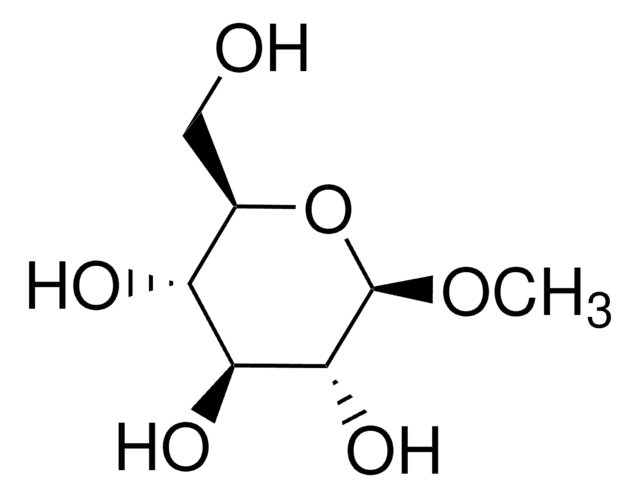
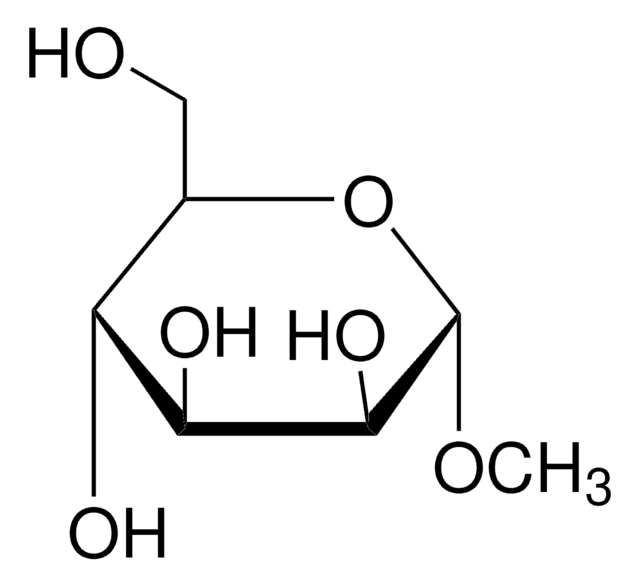
Methyl-β-D-galactopyranoside
Synonym(s):
Methyl β-D-galactoside
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14O6
CAS Number:
Molecular Weight:
194.18
Beilstein:
81569
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥98% (GC)
form
powder
technique(s)
gas chromatography (GC): suitable
color
white to faint yellow
mp
176-179 °C
solubility
water: 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3-,4+,5+,6-,7-/m1/s1
InChI key
HOVAGTYPODGVJG-VOQCIKJUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
A β-D-galactopyranoside having a methyl substituent at the anomeric position.
Biochem/physiol Actions
Methyl galactoside is a hexose involved in the metabolism of 2-deoxygalactose.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Feng Yang et al.
Carbohydrate research, 337(6), 485-491 (2002-03-14)
Oligosaccharide derivatives from sanqi, a Chinese herbal medicine derived from Panax notoginseng, methyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, diosgenyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, and methyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranosyl-(1-->4)-beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, were synthesized under standard glycosylation conditions. An unexpected alpha-(1-->4) linkage was formed predominantly in the presence of neighboring participation group during
Joel S Griffitts et al.
Science (New York, N.Y.), 307(5711), 922-925 (2005-02-12)
The development of pest resistance threatens the effectiveness of Bacillus thuringiensis (Bt) toxins used in transgenic and organic farming. Here, we demonstrate that (i) the major mechanism for Bt toxin resistance in Caenorhabditis elegans entails a loss of glycolipid carbohydrates;
J W Dallinga et al.
Biological mass spectrometry, 23(12), 764-770 (1994-12-01)
The fast atom bombardment collision-induced dissociation mass spectra of the [M-H]- ions of the 2-, 3-, 4- and 6-deoxy derivatives from methyl beta-D-galactopyranoside and some related compounds have been recorded. The fragmentation reactions of these quasimolecular ions and of OD-labelled
T J Kelley et al.
Bioscience reports, 19(5), 433-447 (2000-04-14)
The highly purified DNA Pol-alpha from rat prostate tumor (PA-3) and human neuroblastoma (IMR-32) cells appeared to be inhibited by Ricin (RCA-II), and Con-A. Loss of activity (40 to 60%) of a specific form of DNA polymerase from IMR-32 was
Peter Capek
Carbohydrate research, 343(8), 1390-1393 (2008-04-25)
An arabinogalactan with a high content of 3-O-methyl-D-galactose residues has been isolated from the aerial parts of sage (Salvia officinalis L.). Structural studies of the polymer indicated a beta-1,6-D-galactopyranose backbone in which at least one-third of D-galactopyranosyl residues carries methoxyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service