H8125
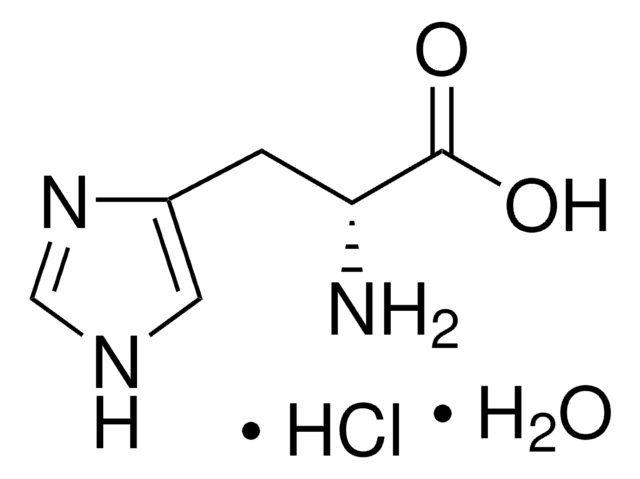
L-Histidine monohydrochloride monohydrate
≥98% (HPLC)
Synonym(s):
L-α-Amino-β-(4-imidazolyl)propionic acid monohydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C6H9N3O2 · HCl · H2O
CAS Number:
Molecular Weight:
209.63
Beilstein:
4168261
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
≥98% (HPLC)
mp
254 °C (dec.) (lit.)
solubility
H2O: 50 mg/mL, clear, colorless to very faintly yellow
SMILES string
O.Cl.N[C@@H](Cc1c[nH]cn1)C(O)=O
InChI
1S/C6H9N3O2.ClH.H2O/c7-5(6(10)11)1-4-2-8-3-9-4;;/h2-3,5H,1,7H2,(H,8,9)(H,10,11);1H;1H2/t5-;;/m0../s1
InChI key
CMXXUDSWGMGYLZ-XRIGFGBMSA-N
Looking for similar products? Visit Product Comparison Guide
Application
L-Histidine monohydrochloride monohydrate has been used as a component of broth to match the tuna meat amino acid composition. It has also been used for partial rescue of the IMPL2 (myo-inositol monophosphatase) phenotype during seed development of Arabidopsis.
Biochem/physiol Actions
Histidine takes part in protein methylation. It is associated with the hemoglobin structure and function. Histidine is present in antioxidative dipeptides. It is involved in one-carbon unit metabolism.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Modelling the effect of temperature, carbon dioxide, water activity and pH on growth and histamine formation by Morganella psychrotolerans
Emborg J and Dalgaard P
International Journal of Food Microbiology, 128(2), 226-233 (2008)
Garrett RH and Grisham CM
Biochemistry (2012)
Kiana Khadem-Abbassi et al.
Nanomaterials (Basel, Switzerland), 9(5) (2019-05-07)
This work describes the preparation of molecularly imprinted polymer (MIP)-modified core/shell CdTe0.5S0.5/ZnS quantum dots (QDs). The QDs@MIP particles were used for the selective and sensitive detection of dopamine (DA). Acrylamide, which is able to form hydrogen bonds with DA, and
Expression and functions of myo-inositol monophosphatase family genes in seed development of Arabidopsis
Sato Y, et al.
Journal of Plant Research, 124(3), 385-394 (2011)
Yuko Sato et al.
Journal of plant research, 124(3), 385-394 (2010-10-21)
Myo-inositol monophosphatase (IMP) catalyzes the dephosphorylation of myo-inositol 3-phosphate in the last step of myo-inositol biosynthesis. IMP is also important in phosphate metabolism and is required for the biosynthesis of cell wall polysaccharides, phytic acid, and phosphatidylinositol. In Arabidopsis, IMP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service