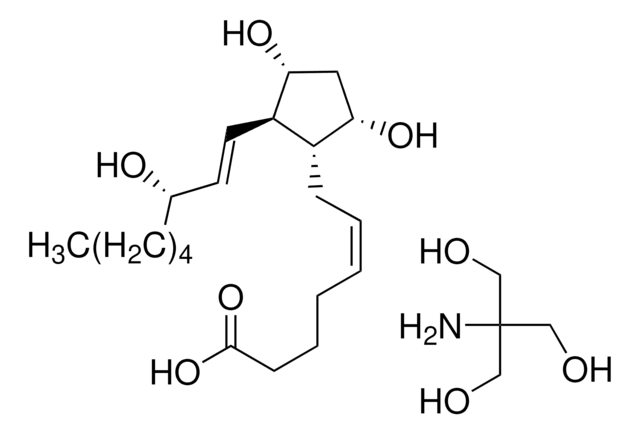
D8174
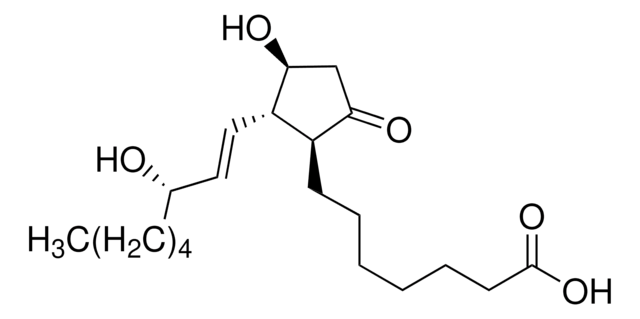
9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α
≥98% (HPLC), solution, thromboxane A2 agonist
Synonym(s):
U-46619, U46619
About This Item
Recommended Products
Product Name
9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α, solution, 10 mg/mL in methyl acetate
form
solution
Quality Level
concentration
10 mg/mL in methyl acetate
shipped in
dry ice
storage temp.
−20°C
SMILES string
CCCCC[C@H](O)\C=C\[C@H]1C2CC(CO2)[C@@H]1C\C=C/CCCC(O)=O
InChI
1S/C21H34O4/c1-2-3-6-9-17(22)12-13-19-18(16-14-20(19)25-15-16)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16?,17-,18-,19+,20?/m0/s1
InChI key
LQANGKSBLPMBTJ-REGKDVDGSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to induce aortic smooth muscle (SM) contraction in mice deficient in myosin light chain 9 (Myl9) gene
- to induce contraction as part of vascular reactivity experiments using mice aorta
- as a thromboxane/prostaglandin agonist to study the effect of dithiothreitol (DTT) on mice arterial vessel viability
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
14.0 °F
Flash Point(C)
-10 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover Bioactive Small Molecules for Lipid Signaling Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service