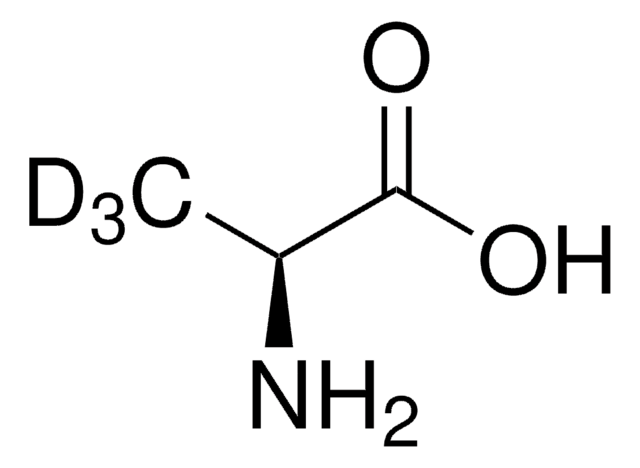
76157
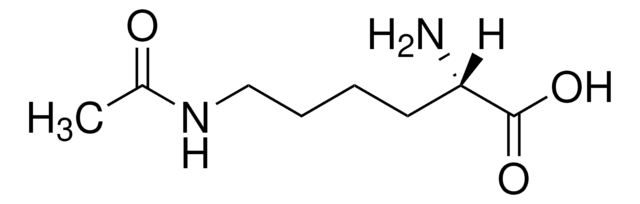
(4R)-4-Hydroxy-L-glutamic acid
≥98.0% (TLC)
Synonym(s):
erythro-(4R)-4-Hydroxy-L-glutamic acid, H-(2S,4R)-γ-Hydroxy-Glu-OH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO5
CAS Number:
Molecular Weight:
163.13
Beilstein:
1725871
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.28
Recommended Products
Product Name
(4R)-4-Hydroxy-L-glutamic acid, ≥98.0% (TLC)
Quality Level
Assay
≥98.0% (TLC)
form
powder
optical activity
[α]/D 20.5±1.5°, c = 1 in H2O
color
white
mp
171 °C
storage temp.
−20°C
SMILES string
N[C@@H](C[C@@H](O)C(O)=O)C(O)=O
InChI
1S/C5H9NO5/c6-2(4(8)9)1-3(7)5(10)11/h2-3,7H,1,6H2,(H,8,9)(H,10,11)/t2-,3+/m0/s1
InChI key
HBDWQSHEVMSFGY-STHAYSLISA-N
Biochem/physiol Actions
(4R)-4-Hydroxy-L-glutamic acid or (2S,4R)-4-hydroxyglutamate was shown to activate the metabotropic glutamate receptors, mGlu1a, mGlu2, and mGlu8a in a dose-dependent manner.
Substrate for aminotransferase; pharmacological characterization at human glutamate transporter subtypes 1-3; for studying structure-activity relationships (SAR) for ionotropic and metabotropic glutamate receptors.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Metabolism of gamma-hydroxyglutamic acid. I. Conversion to alpha-hydroxy-gamma-ketoglutarate by purified glutamic-aspartic transaminase to rat liver.
A GOLDSTONE et al.
The Journal of biological chemistry, 237, 3476-3485 (1962-11-01)
A S Bessis et al.
Bioorganic & medicinal chemistry letters, 11(12), 1569-1572 (2001-06-20)
The (2S,4R)- and (2S,4S)-4-hydroxyglutamates activate cloned mGlu(1a), mGlu(2), and mGlu(8a) receptors with different potencies. Best results were obtained with the (2S,4S) isomer being almost as potent as glutamate on mGlu(1a)R and mGlu(8a)R. Data are interpreted on the basis of the
Lennart Bunch et al.
ChemMedChem, 4(11), 1925-1929 (2009-09-05)
Subtype-selective ligands are of great interest to the scientific community, as they provide a tool for investigating the function of one receptor or transporter subtype when functioning in its native environment. Several 4-substituted (S)-glutamate (Glu) analogues were synthesized, and altogether
Sebastien Alaux et al.
Journal of medicinal chemistry, 48(25), 7980-7992 (2005-12-13)
A series of nine L-2,4-syn-4-alkylglutamic acid analogues (1a-i) were synthesized in high yield and high enantiomeric excess (>99% ee) from their corresponding 4-substituted ketoglutaric acids (2a-i), using the enzyme aspartate aminotransferase (AAT) from pig heart or E. coli. The synthesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service