All Photos(3)
About This Item
Linear Formula:
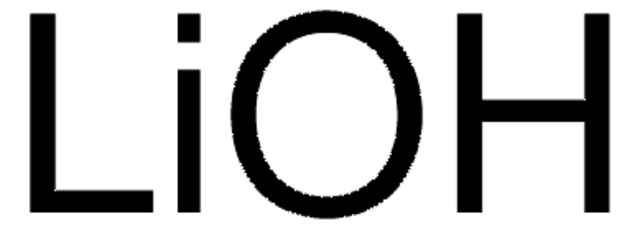
LiOH
CAS Number:
Molecular Weight:
23.95
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.21
Assay:
≥98%
grade:
reagent grade
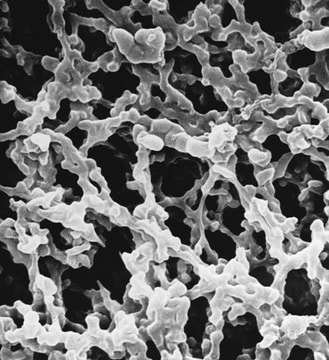
form:
powder
solubility:
water: soluble 71 g/L at 20 °C
Recommended Products
grade
reagent grade
Quality Level
Assay
≥98%
form
powder
mp
470 °C (dec.) (lit.)
solubility
water: soluble 71 g/L at 20 °C
SMILES string
[Li+].[OH-]
InChI
1S/Li.H2O/h;1H2/q+1;/p-1
InChI key
WMFOQBRAJBCJND-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Lithium hydroxide (LiOH) is an alkali metal hydroxide. Lithium chloride solution in water on electrolysis forms LiOH. In respiratory apparatus and submarines, it is utilized to uptake carbon dioxide. A study on the redox mechanism of titanium dioxide (TiO2) using cyclic voltammetry, X-ray diffraction, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) in aqueous LiOH electrolyte has been reported.
Application
Lithium hydroxide has been used in the following processes:
- Synthesis of lithium-doped zinc oxide (ZnO) thin films.
- Preparation of lithium glyceroxide/hydroxide catalysts by reacting with glycerol.
- As a catalyst to generate unsaturated ketones via Michael addition of β-dicarbonyl compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lithium Hydroxide-Catalyzed Conjugate Addition of β-Dicarbonyl Compounds.
Bonadies F, et al.
ChemInform, 26(23) (1995)
G F Zhang et al.
Journal of animal science, 92(7), 2987-2995 (2014-05-08)
The objective of this experiment was to determine the net energy requirements for maintenance of growing and finishing pigs using regression models. Thirty-six growing (27.38 ± 2.24 kg) and 36 finishing (70.25 ± 2.61 kg) barrows were used and within
Eagleson M.
Concise Encyclopedia Chemistry, 605-605 (1994)
Low-Temperature, Solution-Processed and Alkali Metal Doped ZnO for High-Performance Thin-Film Transistors.
Park SY, et al.
Advanced Mat., 24(6), 834-838 (2012)
Electrochemical behavior of anatase TiO2 in aqueous lithium hydroxide electrolyte.
Manickam M, et al.
J. Appl. Electrochem., 36(5), 599-602 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service