PHR2407
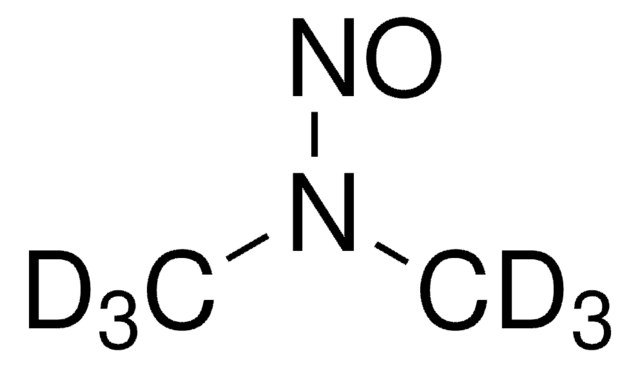
N-Nitrosodimethylamine (NDMA)
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
N-Nitrosodimethylamine, NDMA, Dimethylnitrosamine
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1466674
vapor pressure
5 mmHg ( 20 °C)
form
liquid
CofA
current certificate can be downloaded
packaging
pkg of 100 mg
refractive index
n20/D 1.437 (lit.)
bp
153 °C/774 mmHg (lit.)
density
1.01 g/mL (lit.)
application(s)
pharmaceutical
storage temp.
2-8°C
SMILES string
CN(C)N=O
InChI
1S/C2H6N2O/c1-4(2)3-5/h1-2H3
InChI key
UMFJAHHVKNCGLG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
It is provided with a comprehensive certificate of analysis (CoA) containing a certified purity value, calculated by the mass balance approach. All information regarding the use of this CRM can be found on the certificate of analysis.N-Nitrosodimethylamine (NDMA) is a nitrosamine that occurs as an impurity in sartan angiotensin II receptor blocker drugs.
Application
- Determination of N-Nitrosodimethylamine (NDMA) as an impurity in four valsartan APIs and tablets by high-performance liquid chromatography (HPLC)
- Quantitative analysis of NDMA in valsartan pharmaceutical formulations by capillary electrophoresis-nanospray-mass spectrometry
- Simultaneous determination of N-nitrosodimethylamine and N-nitrosomethylethylamine in drug substances and products containing sartans, ranitidine, and metformin by solid-phase extraction (SPE) and gas chromatography-tandem mass spectrometry (GC-MS/MS)
- Analysis of NDMA in the olmesartan API and tablets by high-performance liquid chromatography-mass spectrometry (HPLC-MS)
- Development and validation of an HPLC-MS/MS method for separation and quantification of NDMA impurity for quality control of ranitidine products
Biochem/physiol Actions
Other Notes
Footnote
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Chronic 2 - Carc. 1B - STOT RE 1
Target Organs
Liver
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
141.8 °F - closed cup
Flash Point(C)
61.0 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Nitrosamines have been discovered as a serious contaminant group in active pharmaceutical ingredients (API) belonging to the sartan family. This article describes a GC-MS method for the determination of nitrosamines in Valsartan tablets according to US FDA guide lines that can be used for pharma QC.
Learn about LC-MS/MS method development to quantify NDMA impurity in valsartan drug substance using Titan™ C18 column based UHPLC separation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service