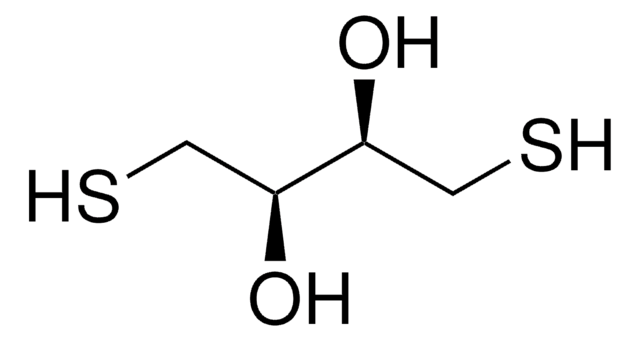
D5545
DL-Dithiothreitol
BioXtra, ≥99.0% (titration)
Synonym(s):
threo-1,4-Dimercapto-2,3-butanediol, Cleland’s reagent, DTT
About This Item
Recommended Products
product line
BioXtra
Quality Level
Assay
≥99.0% (titration)
form
powder
reaction suitability
reagent type: reductant
impurities
Insoluble matter, passes filter test
≤0.3% 4,5-dihydroxy-1,2-dithiane
pH
4.0-6.0 (20-25 °C, 0.1 m in H2O)
mp
41-44 °C (lit.)
solubility
H2O: 0.1 M, clear, colorless
anion traces
sulfate (SO42-): ≤0.005%
cation traces
Al: ≤0.0005%
As: ≤0.0001%
Ba: ≤0.0005%
Bi: ≤0.0005%
Ca: ≤0.001%
Cd: ≤0.0005%
Co: ≤0.0005%
Cr: ≤0.0005%
Cu: ≤0.0005%
Fe: ≤0.0005%
K: ≤0.005%
Li: ≤0.0005%
Mg: ≤0.0005%
Mn: ≤0.0005%
Mo: ≤0.0005%
Na: ≤0.1%
Ni: ≤0.0005%
Pb: ≤0.0005%
Sr: ≤0.0005%
Zn: ≤0.0005%
absorption
≤0.100 at 280 in H2O at 0.1 M
≤0.400 at 260 in H2O at 0.1 M
storage temp.
2-8°C
SMILES string
O[C@H](CS)[C@H](O)CS
InChI
1S/C4H10O2S2/c5-3(1-7)4(6)2-8/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
VHJLVAABSRFDPM-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Step-by-step workflows for the intact mass analysis, peptide mapping, and N-glycan analysis of the monoclonal antibody― adalimumab, for an accurate characterization of the critical quality attributes (CQAs) to ensure drug safety and efficacy. Read more.
Protocols
Step-by-step SEC-MS protocol for rapid glycoprofiling of a monoclonal antibody (mAb), consisting of antibody purification, a mAb reduction procedure, a mass spectrometer calibration, and a system suitability test.
A complete workflow for the intact and middle-up mass analysis of reduced and non-reduced monoclonal antibodies based on SEC-MS with sample preparation by protein-A affinity clean-up.
Related Content
Step-by-step reversed phase UHPLC-MS workflow for middle-up mass analysis of an immunoglobulin G antibody, consisting of antibody purification, IdeS proteolysis and reduction, mass spectrometer calibration, mAb quantification, and a system suitability test.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service