L-019
Lamotrigine solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
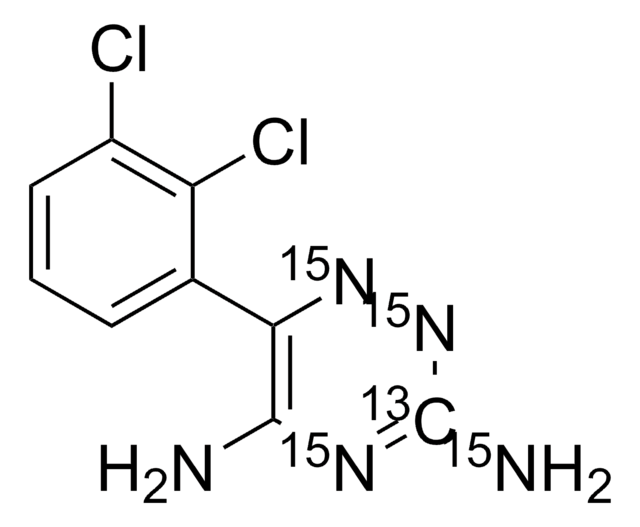
SMILES string
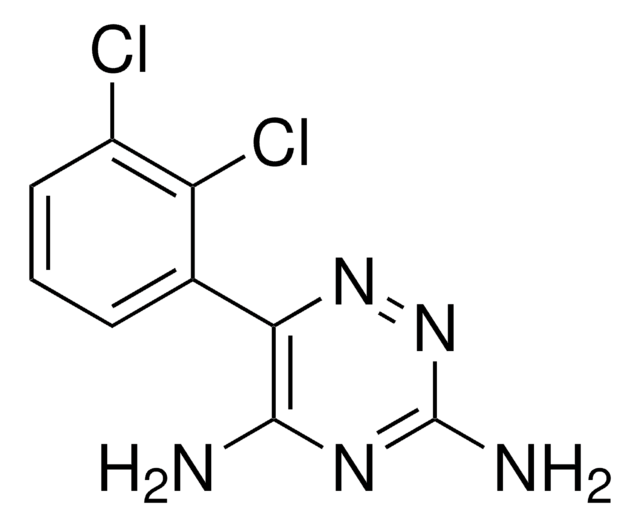
Nc1nnc(c(N)n1)-c2cccc(Cl)c2Cl
InChI
1S/C9H7Cl2N5/c10-5-3-1-2-4(6(5)11)7-8(12)14-9(13)16-15-7/h1-3H,(H4,12,13,14,16)
InChI key
PYZRQGJRPPTADH-UHFFFAOYSA-N
Gene Information
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
General description
Application
- Metabolic Impact in Bipolar Disorder: Lamotrigine is studied for its metabolic effects when used alongside antipsychotics in bipolar disorder management. The research, conducted through a network meta-analysis, provides insights into the mood stabilization and metabolic considerations during treatment (Kong et al., 2024).
- Forensic Application: Lamotrigine′s quantification in human plasma is critical in forensic science for ensuring accurate therapeutic monitoring and legal investigations. This application leverages HPLC-PDA techniques to analyze forensic samples, demonstrating the drug′s significance beyond clinical settings (Sánchez-Sellero et al., 2024).
- Advanced Drug Monitoring: A study has developed a method using LC-MS/MS for the simultaneous monitoring of lamotrigine among other antiepileptic drugs through dried blood and plasma spot sampling. This technique enhances the pharmacokinetic analysis and is pivotal for patient management in epilepsy treatment (Cao et al., 2024).
- Innovative Extraction Techniques: Research has introduced a molecularly imprinted polymer membrane for the selective extraction of lamotrigine, showcasing its utility in separating and quantifying this drug alongside other pharmaceuticals. This innovative approach benefits various biochemical assays and enhances drug analysis capabilities (Zhao et al., 2024).
- Enhanced Analytical Methodology: Lamotrigine′s determination in urine samples through a titania-based fabric phase sorptive extraction reflects its critical role in therapeutic drug monitoring. This method, paired with high-performance liquid chromatography, offers precise measurements essential for neurological disorder management (Ulusoy et al., 2024).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service