791300P
Avanti
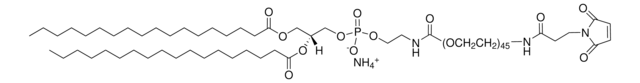
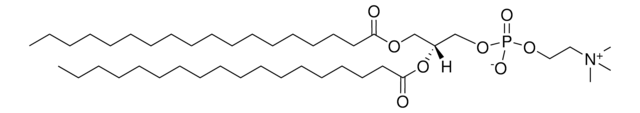
18:0 PE-DTPA
Avanti Research™ - A Croda Brand 791300P, powder
Synonym(s):
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (ammonium salt)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C55H118N9O17P
CAS Number:
Molecular Weight:
1208.55
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 5 mg (791300P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 791300P
shipped in
dry ice
storage temp.
−20°C
General description
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (18:0 PE-DTPA) comprises synthetic phospholipid derivative of ethanolamine.
This chelate is used for preparing MRI contrast agents.
Application
18:0 PE-DTPA (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (ammonium salt)) has been used to chelate liposome-based cationic adjuvant formulation (CAF01). It has also been used in the liposome preparation for imaging studies
Biochem/physiol Actions
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (18:0 PE-DTPA) or PE-DTPA binding and interaction with the Toll-like receptor-2 regulates immune response . PE-DTPA acts as a lipopolysaccharide antagonist and elicits rescue functionality in sepsis by blocking nuclear factor κ -light-chain-enhancer of activated B cells based transcription.
Packaging
5 mL Amber Glass Screw Cap Vial (791300P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Brian Mog et al.
Nanotheranostics, 3(4), 342-355 (2019-11-15)
Specific targeting of inflammation remains a challenge in many pathologies, because of the necessary balance between host tolerance and efficacious inflammation resolution. Here, we discovered a short, 4-mer peptide which possesses antagonist properties towards CC chemokine receptor 2 (CCR2), but
Zahraa S Al-Ahmady et al.
ACS nano, 13(11), 12470-12486 (2019-11-07)
The development of effective therapies for stroke continues to face repeated translational failures. Brain endothelial cells form paracellular and transcellular barriers to many blood-borne therapies, and the development of efficient delivery strategies is highly warranted. Here, in a mouse model
Jin Young Kang et al.
Immunity, 31(6), 873-884 (2009-11-26)
Toll-like receptor 2 (TLR2) initiates potent immune responses by recognizing diacylated and triacylated lipopeptides. Its ligand specificity is controlled by whether it heterodimerizes with TLR1 or TLR6. We have determined the crystal structures of TLR2-TLR6-diacylated lipopeptide, TLR2-lipoteichoic acid, and TLR2-PE-DTPA
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service