M14943
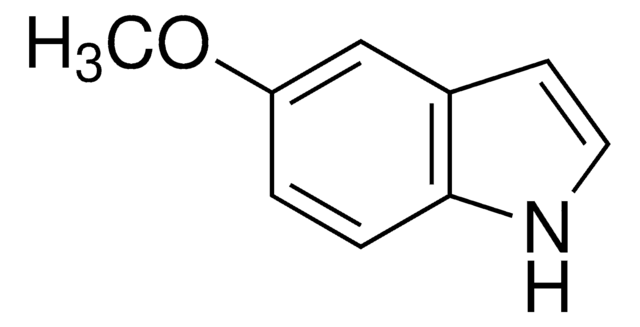
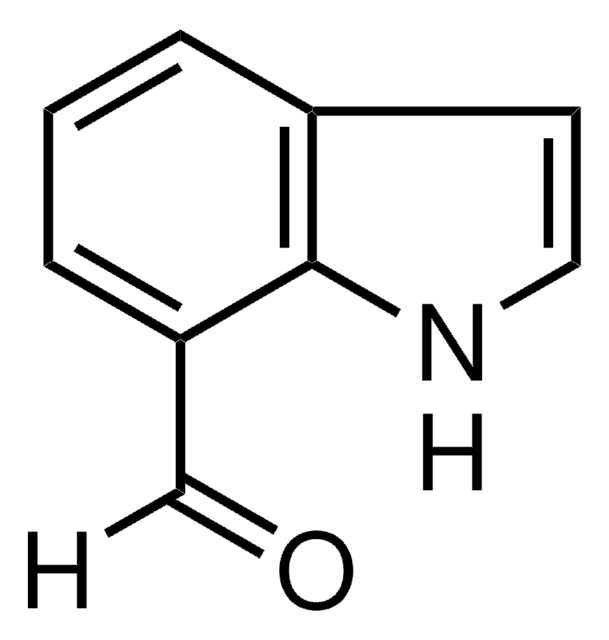
5-Methoxyindole-3-carboxaldehyde
≥99%
Synonym(s):
3-Formyl-5-methoxyindole, NSC 521754
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9NO2
CAS Number:
Molecular Weight:
175.18
Beilstein:
132769
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
mp
179-183 °C (lit.)
SMILES string
COc1ccc2[nH]cc(C=O)c2c1
InChI
1S/C10H9NO2/c1-13-8-2-3-10-9(4-8)7(6-12)5-11-10/h2-6,11H,1H3
InChI key
TUWARWGEOHQXCO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- reactant in synthesis of tryptophan dioxygenase inhibitors as potential anticancer immunomodulators
- reactant in preparation of inhibitor of the C-terminal domain of RNA polymerase II
- reactant in preparation of imidazopyridines and imidazobenzothiazoles
- reactant in preparation of fluorescent neuroactive probes for brain imaging
- reactant in preparation of antibacterial agents
- reactant in synthesis of antiandrogens
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jinming Zhou et al.
Investigational new drugs, 28(3), 291-298 (2009-04-25)
A crucial event in prostate cancer progression is the transition from a hormone-sensitive to a lethal castration-refractory disease state. The antagonist-to-agonist conversion due to mutation in AR is a critical problem with the current clinically used antiandrogens. We aim to
Adrienne S Brown et al.
Organic & biomolecular chemistry, 9(7), 2142-2148 (2011-02-05)
A set of spectrally diverse stilbazolium dyes was identified in an uptake assay using cultured brainstem and cerebellum cells isolated from e19 chicks. Pretreatment of cells with indatraline, a monoamine reuptake inhibitor, allowed identification of dyes that may interact with
Shuhong Wu et al.
Journal of medicinal chemistry, 54(8), 2668-2679 (2011-03-30)
To optimize the antitumor activity of oncrasin-1, a small molecule RNA polymerase II inhibitor, we evaluated 69 oncrasin-1 analogues for their cytotoxic activity against normal human epithelial cells and K-Ras mutant tumor cells. About 40 of those compounds were as
Taleb H Al-Tel et al.
European journal of medicinal chemistry, 46(5), 1874-1881 (2011-03-19)
New antimicrobial agents, imidazo[1,2-a]pyridine and imidazo[2,1-b][1,3]benzothiazole, have been synthesized. Their antimicrobial activities were conducted against various Gram-positive, Gram-negative bacteria and fungi. Compounds 6c, 7a, 10b, 11a, 12b, 14a, 14b, 15a and 15b, exerted strong inhibition of the investigated bacterial and
Anas J M Rasras et al.
European journal of medicinal chemistry, 45(6), 2307-2313 (2010-02-26)
Synthesis and antimicrobial activity of cholic acid analogues 4a-t are reported. The synthesis of 4a-t was accomplished from ethylcholate 2. The hydrazone moiety was introduced via coupling of the cholic acid hydrazide (3) with appropriately functionalized aldehyde utilizing acetic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1H-Benzo[g]indole 97%](/deepweb/assets/sigmaaldrich/product/structures/568/798/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd/640/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd.png)