M0505
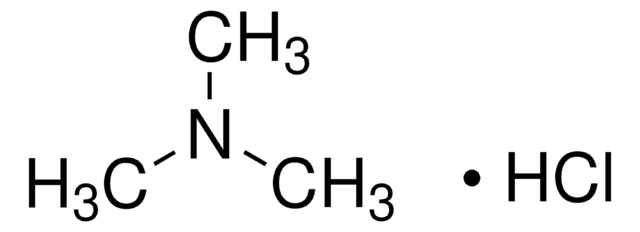
Methylamine hydrochloride
≥98%
Synonym(s):
Methanaminium chloride, Methylamine monohydrochloride, Methylammonium chloride, Monomethylammonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3NH2 · HCl
CAS Number:
Molecular Weight:
67.52
Beilstein:
3588822
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder or crystals
bp
225-230 °C/15 mmHg (lit.)
mp
231-233 °C (lit.)
solubility
H2O: 1 g in 10 mL, clear, colorless
SMILES string
Cl[H].CN
InChI
1S/CH5N.ClH/c1-2;/h2H2,1H3;1H
InChI key
NQMRYBIKMRVZLB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methylamine hydrochloride is used as a buildingblock for the synthesis of various organic compounds.
Application
Methylamine hydrochloride can be used as a reactant to synthesize:
- Betahistine via aza-Michael reaction with 2-vinylpyridine in water.
- Azatripyrrolic and azatetrapyrrolic macrocycles by reacting with pyrrole and formaldehyde via base-catalyzed Mannich reaction.
- Tetrahydropyridines via Aza-Diels–Alder reaction with dienes and aldehydes.
- N-methylsecondary arylamines from aryl chlorides via nickel-catalyzed amination reaction.
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
408.7 °F - closed cup
Flash Point(C)
209.3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marco Dionisio et al.
Journal of the American Chemical Society, 134(15), 6540-6543 (2012-04-06)
Single-walled carbon nanotubes (SWCNTs) have been functionalized with highly selective tetraphosphonate cavitand receptors. The binding of charged N-methylammonium species to the functionalized SWCNTs was analyzed by X-ray photoelectron spectroscopy and confirmed by (31)P MAS NMR spectroscopy. The cavitand-functionalized SWCNTs were
Elisabeth Schwaiger et al.
Transplantation, 97(12), 1279-1285 (2014-03-14)
Luminex-based anti-HLA IgG detection on single-antigen flow beads (SAFB) represents a valuable tool for characterization of allosensitization patterns. Assay interpretation, however, may be impeded by false-low test results caused by the prozone effect. Recent experimental data have related this artifact
Jing-Jing Fang et al.
Waste management (New York, N.Y.), 32(7), 1401-1410 (2012-04-07)
This study investigated the odor compounds from different areas in a landfill site, which included the municipal solid waste (MSW)-related area, the leachate-related area and the sludge-related area. Nine sampling points were placed and 35 types of odorous substances were
Nicolas Fleury-Brégeot et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(31), 9564-9570 (2012-07-07)
Ammoniomethyl trifluoroborates are very powerful reagents that can be used to access biologically relevant aryl- and heteroaryl-methylamine motifs via Suzuki-Miyaura cross-couplings. Until now, this method was limited to the production of tertiary and primary amines. The synthesis of a large
Raymond J Ritchie
Microbial ecology, 65(1), 180-196 (2012-09-04)
Ammonia is the preferred nitrogen source for many algae including the cyanobacterium Synechococcus elongatis (Synechococcus R-2; PCC 7942). Modelling ammonia uptake by cells is not straightforward because it exists in solution as NH(3) and NH (4) (+) . NH(3) is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service