D39169
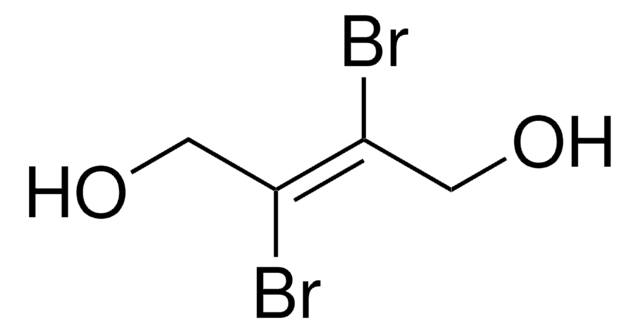
1,4-Dibromo-2,3-butanedione
99%
Synonym(s):
1,4-Dibromodiacetyl
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrCH2COCOCH2Br
CAS Number:
Molecular Weight:
243.88
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
117-119 °C (lit.)
SMILES string
BrCC(=O)C(=O)CBr
InChI
1S/C4H4Br2O2/c5-1-3(7)4(8)2-6/h1-2H2
InChI key
RZMOICJDRADLCT-UHFFFAOYSA-N
Application
- Catalyst-Free Multicomponent Cyclopolymerizations: A novel approach utilizing 1,4-Dibromo-2,3-butanedione in catalyst-free multicomponent cyclopolymerizations to synthesize functional polyiminofurans containing bromomethyl groups was developed. This method enhances the utility of 1,4-Dibromo-2,3-butanedione in producing advanced materials with potential applications in chemical and pharmaceutical industries (Zhu et al., 2021).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chui-Shan Tsang et al.
Chemical communications (Cambridge, England), (15)(15), 1999-2001 (2009-04-01)
Reaction of a pinene-based pyridylthioamide with 1,4-dibromo-2,3-butanedione in refluxing methanol yielded a new chiral pyridylthiazole ligand L which forms a dinuclear double-stranded helicate with Cu(i) ions; this helicate has opposite helical chirality when compared with its quaterpyridine analogue.
S H Vollmer et al.
Protein science : a publication of the Protein Society, 1(5), 678-687 (1992-05-01)
The bifunctional reagent 1,4-dibromobutanedione (DBBD) reacts covalently with pyruvate kinase from rabbit muscle to cause inactivation of the enzyme at a rate that is linearly dependent on the reagent concentration, giving a second order rate constant of 444 min-1 M-1.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service