All Photos(1)
About This Item
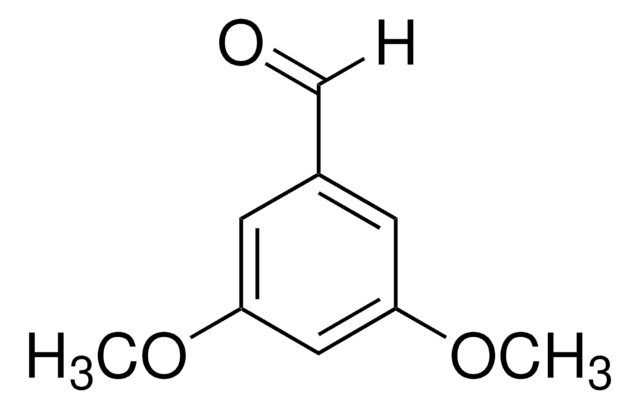
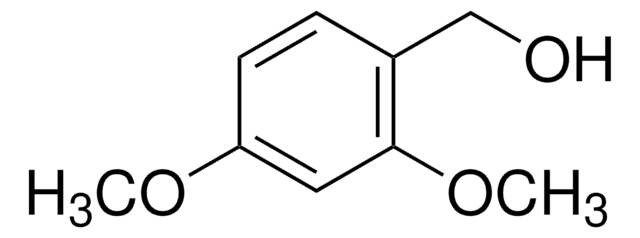
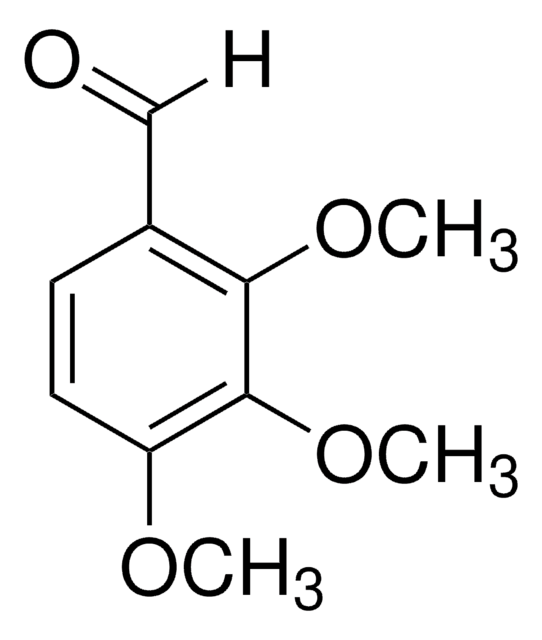
Linear Formula:
(CH3O)2C6H3CHO
CAS Number:
Molecular Weight:
166.17
Beilstein:
607989
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
165 °C/10 mmHg (lit.)
mp
67-69 °C (lit.)
SMILES string
COc1ccc(C=O)c(OC)c1
InChI
1S/C9H10O3/c1-11-8-4-3-7(6-10)9(5-8)12-2/h3-6H,1-2H3
InChI key
LWRSYTXEQUUTKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Francesca Rosi et al.
Analytical and bioanalytical chemistry, 409(12), 3187-3197 (2017-03-08)
A new analytical approach, based on micro-transflection measurements from a diamond-coated metal sampling stick, is presented for the analysis of painting varnishes. Minimally invasive sampling is performed from the varnished surface using the stick, which is directly used as a
Outi Keinänen et al.
Nuclear medicine and biology, 67, 27-35 (2018-11-01)
18F-fluoroglycosylation via oxime formation is a chemoselective and mild radiolabeling method for sensitive molecules. Glycosylation can also improve the bioavailability, in vivo kinetics, and stability of the compound in blood, as well as accelerate clearance of biomolecules. A typical synthesis
J Blaakmeer et al.
International journal of peptide and protein research, 37(6), 556-564 (1991-06-01)
The synthesis of the model compound Aloc-Ala-Ala-Dma-Ala-Ala-OMe has been described as an illustration of the fact that a large group reversibly alkylating the amido group of an oligomer can disturb the regularity of a peptide backbone, oppose its aggregation and
Lidia Montero et al.
Journal of chromatography. A, 1428, 115-125 (2015-07-27)
In the present work, the phlorotannin composition of different Sargassum muticum samples collected at different locations along the North Atlantic coasts as well as the bioactivities related to these components were investigated. After pressurized liquid extraction, the samples collected at
Gustavo P B Carretero et al.
Biochimica et biophysica acta. Biomembranes, 1860(8), 1502-1516 (2018-05-12)
Antimicrobial peptides (AMPs) work as a primary defense against pathogenic microorganisms. BP100, (KKLFKKILKYL-NH2), a rationally designed short, highly cationic AMP, acts against many bacteria, displaying low toxicity to eukaryotic cells. Previously we found that its mechanism of action depends on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service