C11081
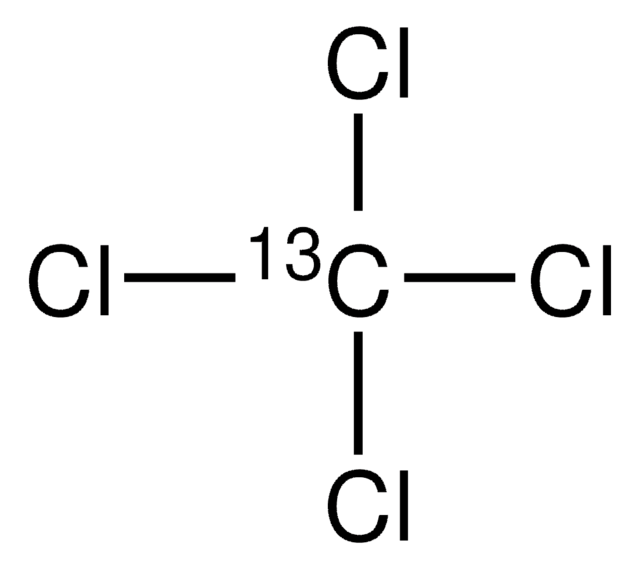
Tetrabromomethane
ReagentPlus®, 99%
Synonym(s):
Carbon tetrabromide
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
CBr4
CAS Number:
Molecular Weight:
331.63
Beilstein:
1732799
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
40 mmHg ( 96 °C)
Quality Level
product line
ReagentPlus®
Assay
99%
form
crystals
bp
190 °C (lit.)
mp
88-90 °C (lit.)
SMILES string
BrC(Br)(Br)Br
InChI
1S/CBr4/c2-1(3,4)5
InChI key
HJUGFYREWKUQJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tetrabromomethane can serve as a mediator, catalyst, and reagent in the synthesis of valuable chemicals.
Application
Tetrabromomethane may be used in the following applications:
- As a transfer agent for the copolymerization of methylmethacrylate and p-divinylbenzene to form soluble crosslinked polymers.
- As a catalyst for the aerobic photooxidative synthesis of aromatic esters from benzyl alcohols under metal-free conditions.
- Bromination of adamantane and its derivatives in the presence of iron compounds as catalyst.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of soluble microgel by the copolymerization of methylmethacrylate with p?divinylbenzene in the presence of tetrabromomethane.
Chen H
Journal of Polymer Science Part A: Polymer Chemistry, 22(9), 2123-2130 (1984)
Aerobic oxidative esterification of benzyl alcohols with catalytic tetrabromomethane under visible light irradiation.
Nobuta T
Tetrahedron Letters, 53(39), 5306-5308 (2012)
Bromination of adamantane and its derivatives with tetrabromomethane catalyzed by iron compounds.
Khusnutdinov RI
Russ. J. Org. Chem., 51(2), 184-187 (2015)
Wendan Cheng et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(26), 6880-6887 (2006-06-23)
Electronic origin for nonresonant enhancement of nonlinear optical response in the complexes formed from tetraalkylammonium halide and carbon tetrabromide is provided in view of electrostatic potentials of intermolecular donor (halide ion)-acceptor (CBr(4)). The calculated electrostatic potentials of donor-acceptor range from
O Salehzadeh et al.
Nanotechnology, 22(16), 165603-165603 (2011-03-12)
Carbon is a commonly used p-type dopant in planar III-V semiconductors, however its use in nanowire (NW) growth has been much less reported. In this work we show that the morphology of gold assisted GaAs NWs can be strongly modified
Global Trade Item Number
| SKU | GTIN |
|---|---|
| C11081-10KG | |
| C11081-100G | 4061833460757 |
| C11081-500G | 4061833460764 |
| C11081-5G | 4061833460771 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service