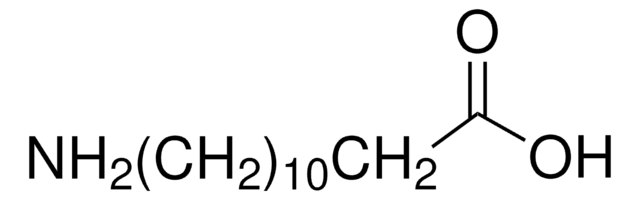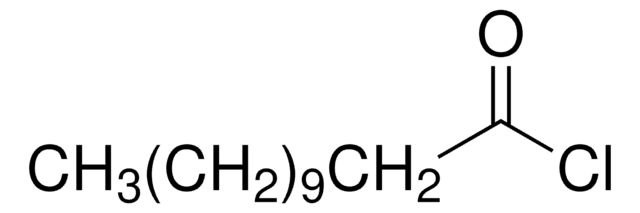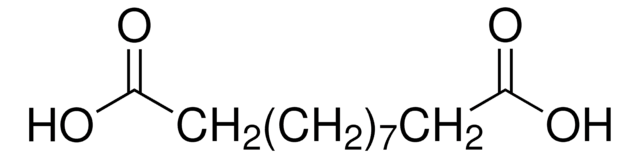A82605
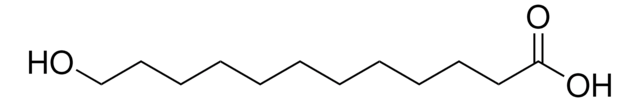
11-Aminoundecanoic acid
97%
Synonym(s):
Aminoundecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2(CH2)10CO2H
CAS Number:
Molecular Weight:
201.31
Beilstein:
1767291
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
white
mp
188-191 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCCCCCCCC(O)=O
InChI
1S/C11H23NO2/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h1-10,12H2,(H,13,14)
InChI key
GUOSQNAUYHMCRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
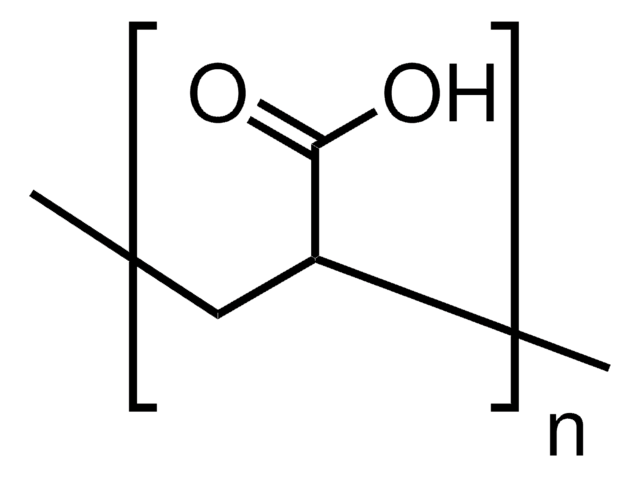
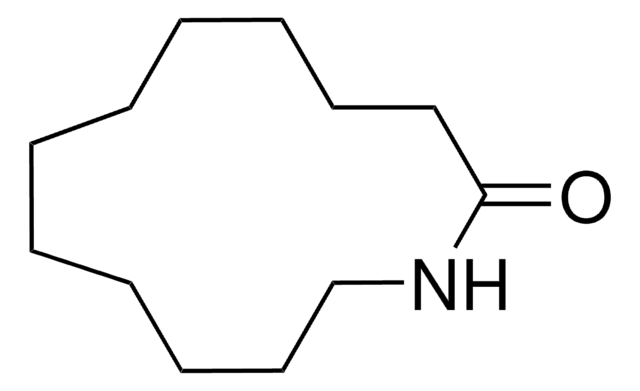
11-Aminoundecanoic acid also known as aminoundecanoic acid, is utilized in solution phase peptide synthesis. It is also a monomer precursor for nylon-11.
Application
11-Aminoundecanoic acid can be used as a linker to synthesize amide-linked linear guanosine dimer.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
DNA binding assay with 11-aminoundecanoic acid-[11-14C] in F-344 rats.
H Peter et al.
Archives of toxicology, 61(1), 86-87 (1987-01-01)
[Antibacterial effect of an ammonium salt of 11-aminoundecanoic acid. 5. Organic ammonium salts].
F Devínsky et al.
Die Pharmazie, 34(9), 574-574 (1979-01-01)
J K Dunnick et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 3(6), 614-618 (1983-11-01)
11-Aminoundecanoic acid, the monomer of nylon 11, was toxic to the urinary tract of both male and female B6C3F1 mice and Fischer 344 rats, when administered in the diet at 7500 or 15 000 ppm for 103-104 weeks. Dose-related effects
E J Matthews
Environmental health perspectives, 101 Suppl 2, 311-318 (1993-07-01)
A co-culture clonal survival assay was developed to measure the cytotoxicity of test chemical treatments to BALB/c-3T3 cells because the standard clonal survival assay using 200 wild type (WT) cells frequently overestimates chemical cytotoxicity when compared with identical treatment doses
Zhiyong Qian et al.
Biomaterials, 25(11), 1975-1981 (2004-01-27)
In this paper, a new kind of aliphatic biodegradable polyesteramide copolymers P(CL/AU)x/y based on epsilon-caprolactone and 11-aminoundecanoic acid were synthesized by the melt polycondensation method. Hydrolytic degradation behavior of P(CL/AU) copolymers were studied by using FTIR, 1H-NMR and DSC. Chemical
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service