851450
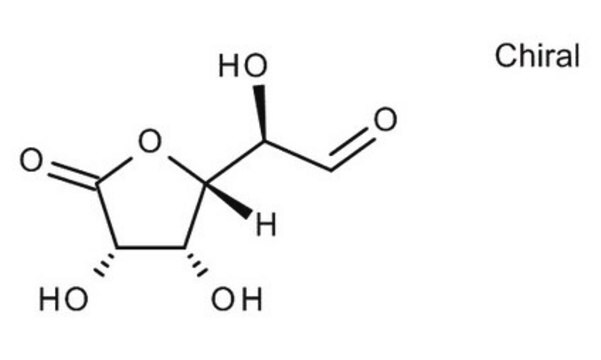
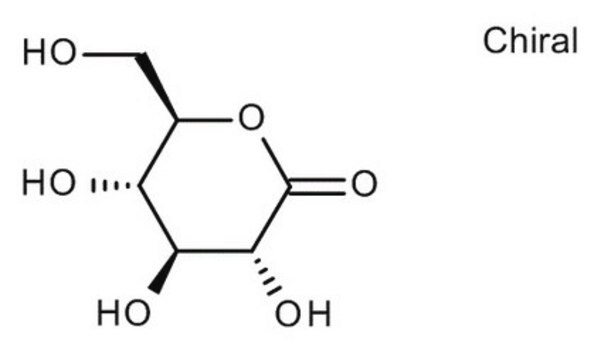
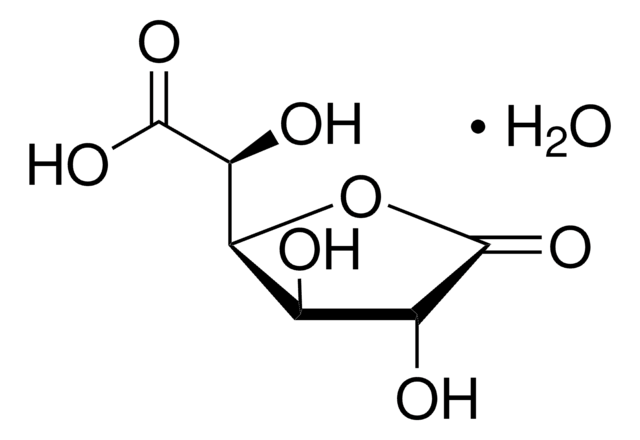
D-(+)-Glucuronic acid γ-lactone
≥99%
Synonym(s):
D-(+)-Glucurono-6,3-lactone, D-Glucurone, D-Glucurono-6,3-lactone, Glucuronolactone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
83595
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
powder
optical activity
[α]24/D +18.8°, c = 8 in H2O
mp
172-175 °C (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless
SMILES string
O=C([C@@H]([C@@H](O1)[C@H](O)[C@H](O)C1=O)O)[H]
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h1-5,8-10H/t2-,3+,4-,5+/m0/s1
InChI key
UYUXSRADSPPKRZ-SKNVOMKLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
D-(+)-Glucuronic acid γ-lactone (Glucourono-γ-lactone, Glucurone or Glycurone) is a carbohydrate derivative. It converted into L-ascorbic acid in animals and human body. Its molecule contains two five-membered rings. Its crystal structure has been studied.
Application
D-(+)-Glucuronic acid γ-lactone may be used in the following studies:
- As starting ragent in the synthesis of 2,3,4,-tris(tert.-butyldimethysilyl) glucuronic acid trichloroethylester, required for the preparation of 1-O-acyl glucuronide of the anti-inflammatory drug ML-3000.
- Synthesis of optically active glucopyranoses.
- Synthesis of long-chain alkyl glucofuranosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Alford et al.
Amino acids, 21(2), 139-150 (2001-10-23)
The effects of Red Bull Energy Drink, which includes taurine, glucuronolactone, and caffeine amongst the ingredients, were examined over 3 studies in a total of 36 volunteers. Assessments included psychomotor performance (reaction time, concentration, memory), subjective alertness and physical endurance.
Rec. Trav. Chim., 113, 79-79 (1994)
Benjamin J Ayers et al.
The Journal of organic chemistry, 77(18), 7777-7792 (2012-08-30)
The enantiomers of glucuronolactone are excellent chirons for the synthesis of the 10 stereoisomeric 2,5-dideoxy-2,5-iminohexitols by formation of the pyrrolidine ring by nitrogen substitution at C2 and C5, with either retention or inversion of configuration; the stereochemistry at C3 may
Andreas F G Glawar et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(30), 9341-9359 (2012-06-28)
The efficient scalable syntheses of 2-acetamido-1,2-dideoxy-D-galacto-nojirimycin (DGJNAc) and 2-acetamido-1,2-dideoxy-D-gluco-nojirimycin (DNJNAc) from D-glucuronolactone, as well as of their enantiomers from L-glucuronolactone, are reported. The evaluation of both enantiomers of DNJNAc and DGJNAc, along with their N-alkyl derivatives, as glycosidase inhibitors showed
A one-step C-linked disaccharide synthesis from carbohydrate allylsilanes and tri-O-acetyl-D-glucal.
A de Raadt et al.
Carbohydrate research, 220, 101-115 (1991-11-11)
The reaction of protected glucuronic esters 2 and 7, as well as D-glucuronolactone derivative 11, with (trimethylsilyl)methylmagnesium chloride in ether led to the corresponding stable bis-silyl adducts 3, 8, and 12, respectively. In Peterson-type reactions catalysed with mild acid, these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service