794287
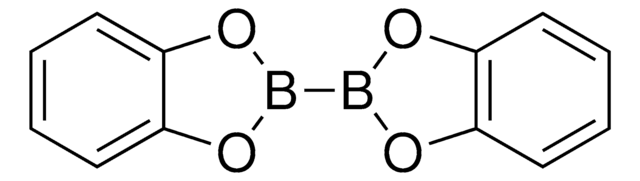
Bis[(pinacolato)boryl]methane
Synonym(s):
2,2′-Methylenebis[4,4,5,5-tetramethyl-1,3,2-dioxaborolane], Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methane
About This Item
Recommended Products
form
solid
Quality Level
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
48 °C
greener alternative category
storage temp.
2-8°C
SMILES string
CC(C(C)(C)O1)(C)OB1CB2OC(C)(C)C(C)(C)O2
InChI
1S/C13H26B2O4/c1-10(2)11(3,4)17-14(16-10)9-15-18-12(5,6)13(7,8)19-15/h9H2,1-8H3
InChI key
MQYZGGWWHUGYDR-UHFFFAOYSA-N
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
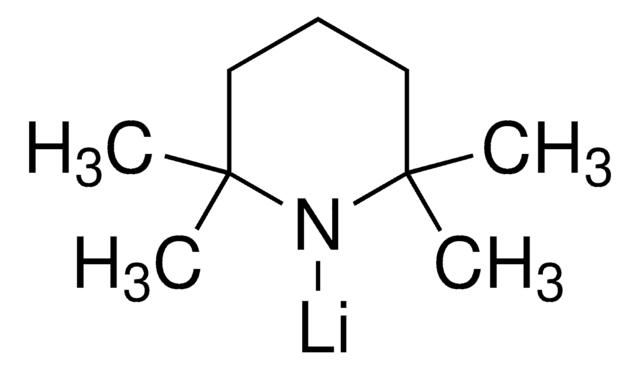
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
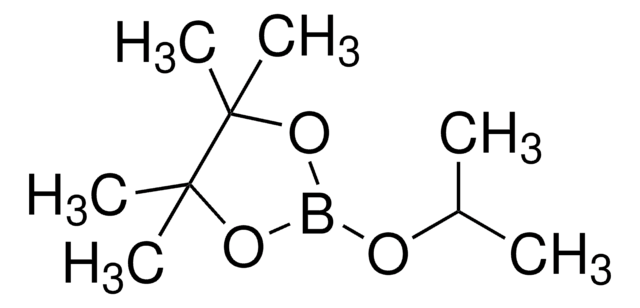
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)