702803
2,1,3-Benzothiadiazole-4,7-bis(boronic acid pinacol ester)
95%
Synonym(s):
Benzo[c]-1,2,3-thiadiazol-4,7-diyl-4,7-diboronic acid dipinacol ester
About This Item
Recommended Products
Quality Level
Assay
95%
form
powder
mp
207-212 °C
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(B3OC(C)(C)C(C)(C)O3)c4nsnc24
InChI
1S/C18H26B2N2O4S/c1-15(2)16(3,4)24-19(23-15)11-9-10-12(14-13(11)21-27-22-14)20-25-17(5,6)18(7,8)26-20/h9-10H,1-8H3
InChI key
DHWADSHFLSDMBJ-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The soaring global demand for energy, coupled with the limited supply of fossil fuels, has increased the need for renewable, low-cost energy sources. Organic electronics have shown great promise for applications in lighting, power, and circuitry, with rapidly improving performance already surpassing that of amorphous silicon-based counterparts.
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-5,10-diboronic acid bis(pinacol) ester 95%](/deepweb/assets/sigmaaldrich/product/structures/396/334/bb0914db-5c9a-4565-ba76-30dd4bc4ba87/640/bb0914db-5c9a-4565-ba76-30dd4bc4ba87.png)
![4,7-Dibromobenzo[c]-1,2,5-thiadiazole 95%](/deepweb/assets/sigmaaldrich/product/structures/711/964/3fd3ffd1-5916-468e-a743-22f1611b5a33/640/3fd3ffd1-5916-468e-a743-22f1611b5a33.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
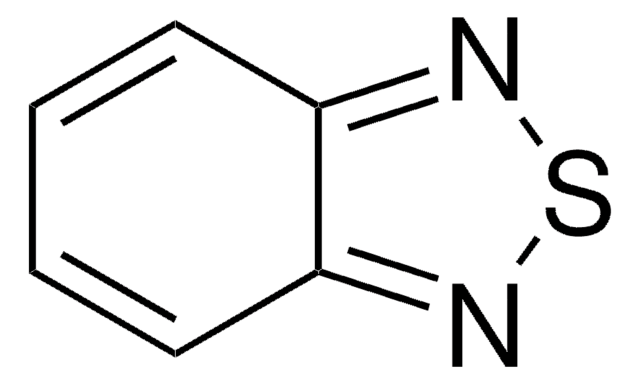
![7-(Dimethylamino)benzo[c][1,2,5]thiadiazole-4-carbaldehyde](/deepweb/assets/sigmaaldrich/product/structures/306/816/20e071cb-86c6-43df-8dcc-24d478208667/640/20e071cb-86c6-43df-8dcc-24d478208667.png)