696188
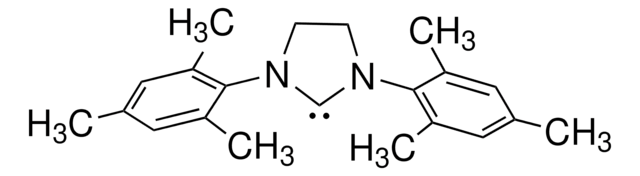
1,3-Bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene
97%
Synonym(s):
1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C21H24N2
CAS Number:
Molecular Weight:
304.43
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
reagent type: catalyst
mp
140 °C
storage temp.
−20°C
SMILES string
Cc1cc(C)c(N2[C]N(C=C2)c3c(C)cc(C)cc3C)c(C)c1
InChI
1S/C21H24N2/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6/h7-12H,1-6H3
InChI key
JCYWCSGERIELPG-UHFFFAOYSA-N
Application
1,3-Bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene (IMes) is a nucleophilic N-heterocyclic carbene (NHC) ligand.
It can be used to synthesize:
IMes is also used as an ancillary ligand in Pd-catalyzed Suzuki-Miyaura cross-coupling reaction between aryl chlorides or aryl triflates and arylboronic acids.
It can be used to synthesize:
- IMes ligated-rhodium complex as a catalyst for the selective hydrogenation of substituted aryl and heteroaryl boronate esters to cis-substituted borylated cycloalkanes.
- IMes/ruthenium complex (Cp*Ru(IMes)Cl)(Cp* =η5-C5Me3) as a catalyst for olefin ring closing metathesis reaction.
IMes is also used as an ancillary ligand in Pd-catalyzed Suzuki-Miyaura cross-coupling reaction between aryl chlorides or aryl triflates and arylboronic acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Olefin metathesis-active ruthenium complexes bearing a nucleophilic carbene ligand
Huang J, et al.
Journal of the American Chemical Society, 121(12), 2674-2678 (1999)
Hydrogenation of (Hetero) aryl Boronate Esters with a Cyclic (Alkyl)(amino) carbene-Rhodium Complex: Direct Access to cis-Substituted Borylated Cycloalkanes and Saturated Heterocycles
Ling L, et al.
Angewandte Chemie (International Edition in English), 58(20), 6554-6558 (2019)
Gilles Schnee et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(49), 17959-17972 (2015-10-21)
The present contribution reports experimental and theoretical mechanistic investigations on a normal-to-abnormal (C2-to-C4-bonded) NHC rearrangement processes occurring with bulky group 13 metal NHC adducts, including the scope of such a reactivity for Al compounds. The sterically congested adducts (nItBu)MMe3 (nItBu=1,3-di-tert-butylimidazol-2-ylidene;
Suzuki- Miyaura cross-coupling reactions mediated by palladium/imidazolium salt systems
Grasa GA, et al.
Organometallics, 21(14), 2866-2873 (2002)
Thi Kim Hoang Trinh et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(39), 9242-9252 (2019-04-26)
In the search of smarter routes to control the conditions of N-heterocyclic carbene (NHCs) formation, a two-component air-stable NHC photogenerating system is reported. It relies on the irradiation at 365 nm of a mixture of 2-isopropylthioxanthone (ITX) with 1,3-bis(mesityl)imidazoli(ni)um tetraphenylborate. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)



![Chloro[1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]gold(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/154/609/370330fe-5c15-47b6-ad13-ea3dc87099d6/640/370330fe-5c15-47b6-ad13-ea3dc87099d6.png)