561657
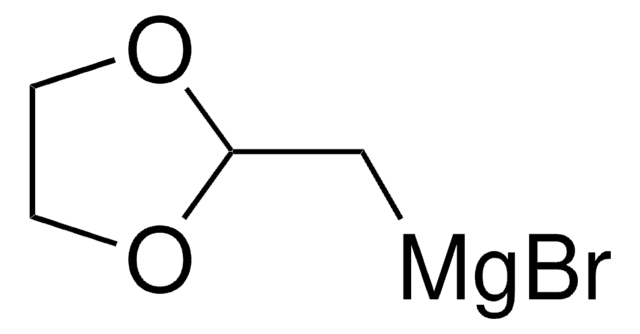
(1,3-Dioxan-2-ylethyl)magnesium bromide solution
0.5 M in THF
Synonym(s):
1,3-Dioxane - 2-ethyl- magnesium complex, [2-(1,3-Dioxan-2-yl)ethyl]magnesium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11BrMgO2
CAS Number:
Molecular Weight:
219.36
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
reaction type: Grignard Reaction
Quality Level
concentration
0.5 M in THF
bp
65 °C
density
0.951 g/mL at 25 °C
functional group
ether
storage temp.
2-8°C
SMILES string
Br[Mg]CCC1OCCCO1
InChI
1S/C6H11O2.BrH.Mg/c1-2-6-7-4-3-5-8-6;;/h6H,1-5H2;1H;/q;;+1/p-1
InChI key
JYNXRXBIEHSSLR-UHFFFAOYSA-M
Related Categories
Application
(1,3-Dioxan-2-ylethyl)magnesium bromide can be used:
- In a Grignard addition-acylation method for the preparation of enamides.
- To prepare trisubstituted allenes by reacting with propargylic ammonium salts.
- In one of the key synthetic steps for the synthesis of febrifugine based antimalarial drugs.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fraser F Fleming et al.
Organic letters, 8(21), 4903-4906 (2006-10-06)
[reaction: see text] Sequential addition of three different Grignard reagents and pivaloyl chloride to 3-oxo-1-cyclohexene-1-carbonitrile installs four new bonds to generate a diverse array of cyclic enamides. Remarkably, formation of the C-magnesiated nitrile intermediate is followed by preferential acylation by
Exploration of a new type of antimalarial compounds based on febrifugine.
Kikuchi H, et al.
Journal of Medicinal Chemistry, 49(15), 4698-4706 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service