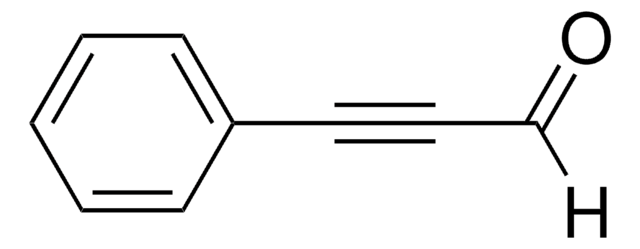
All Photos(1)
About This Item
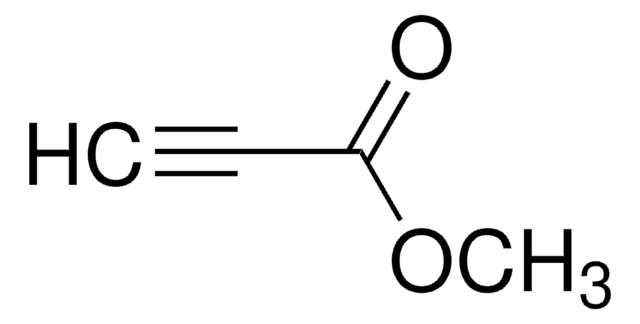
Linear Formula:
C6H5C≡CCO2CH3
CAS Number:
Molecular Weight:
160.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.5590 (lit.)
bp
109-112 °C/2 mmHg (lit.)
density
1.086 g/mL at 25 °C (lit.)
functional group
ester
phenyl
SMILES string
COC(=O)C#Cc1ccccc1
InChI
1S/C10H8O2/c1-12-10(11)8-7-9-5-3-2-4-6-9/h2-6H,1H3
InChI key
JFGWPXKGINUNDH-UHFFFAOYSA-N
Related Categories
General description
Organoiron carbonyl complexes are obtained by reacting methyl phenylpropiolate with Fe2(CO)9.
Application
Methyl phenylpropiolate may be used in the synthesis of:
- bicyclohexadienes
- cis-methyl cinnamate
- (E)-alkyl 3-(dialkoxyphosphoryl)-3-phenylacrylate derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Andrew Evans et al.
Journal of the American Chemical Society, 127(36), 12466-12467 (2005-09-08)
Transition metal-catalyzed [m+n+o] carbocyclization reactions provide powerful methods for the construction of complex polycyclic systems that are generally not accessible through classical pericyclic reactions. We have developed the first regio- and enantioselective crossed intermolecular rhodium-catalyzed [2+2+2] carbocyclization of carbon- and
Preparation and Structures of Methyl Phenylpropiolate-Iron Carbonyl Complexes. A New Dicarbonyl-p-cyclopentadienyloxy-s-vinyliron Compound.
Dahl LF, et al.
Journal of the American Chemical Society, 88(3), 446-452 (1966)
The catalyst-free addition of dialkyl phosphites on the triple bond of alkyl phenylpropiolates under microwave conditions.
Balint E, et al.
Current Catalysis, 4(1), 57-64 (2015)
Steric effects on reactivity in silicon chemistry.
Cartledge FK.
Organometallics, 2(3), 425-430 (1983)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service