542571
Amberlyst® A26 Ion Exchange Resin
hydroxide form, 16-50 mesh
About This Item
Recommended Products
product name
Amberlyst® A26 hydroxide form, hydroxide form
Quality Level
form
beads
concentration
1 N in NaOH (Regenerant concentration)
parameter
60 °C OH- form max. temp.
moisture
66-75%
technique(s)
LPLC: suitable
bed D
≥24 in.
surface area
~30 m2/g
impurities
45-65% (moisture)
matrix
styrene-divinylbenzene (macroreticular)
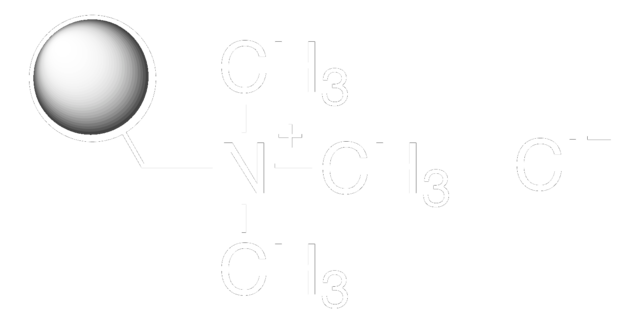
matrix active group
quaternary ammonium functional group
particle size
16-45 mesh
560-700 μm
pore size
0.30 mL/g pore volume
400 Å mean pore size
capacity
0.8 meq/mL by wetted bed volume
bulk density
41 lb/cu.ft
separation technique
anion exchange
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Features and Benefits
Maximum operating temperature 60C.
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service