537349
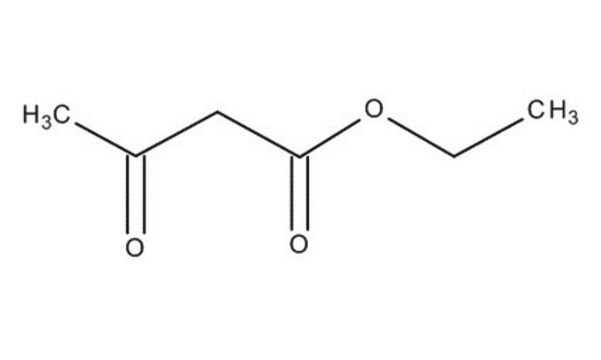
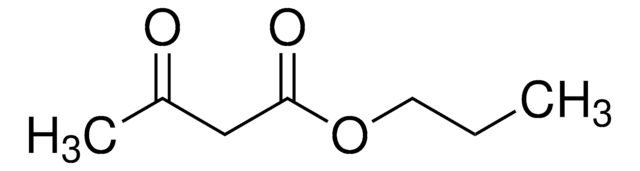
Ethyl acetoacetate
ReagentPlus®, 99%
Synonym(s):
EAA, Ethyl 3-oxobutanoate, Acetoacetic ester
About This Item
Recommended Products
vapor density
4.48 (vs air)
Quality Level
vapor pressure
1 mmHg ( 28.5 °C)
product line
ReagentPlus®
Assay
99%
autoignition temp.
580 °F
expl. lim.
9.5 %
bp
181 °C (lit.)
mp
−43 °C (lit.)
solubility
water: soluble 130 g/L at 20 °C
density
1.029 g/mL at 20 °C (lit.)
functional group
ester
ketone
SMILES string
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
InChI key
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Alkylation at the α-carbon of ethyl acetoacetate followed by hydrolysis and decarboxylation can afford a variety of methyl ketones.
- It also undergoes acylation at the α-carbon in the presence of MgCl2 and pyridine to give synthetically important intermediates.
- It can be used in Knoevenagel condensation with aliphatic, aromatic, and heteroaromatic aldehydes to produce α-alkylideneacetoacetates.
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
164.3 °F - closed cup
Flash Point(C)
73.5 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Biginelli Reaction is an acid-catalyzed, three-component reaction between an aldehyde, b-ketoester, and urea that produces tetrahydropyrimidones, which have potential pharmaceutical applications.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 537349-1KG | 4061832565590 |
| 537349-3KG | 4061832565606 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service