514365
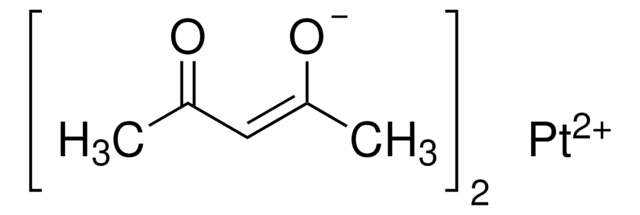
Copper(II) acetylacetonate
≥99.9% trace metals basis
Synonym(s):
2,4-Pentanedione copper(II) derivative, Bis(2,4-pentanedionato)copper(II), Cu(acac)2, Cupric acetylacetonate
About This Item
Recommended Products
Quality Level
Assay
≥99.9% trace metals basis
form
powder
reaction suitability
core: copper
mp
284-288 °C (dec.) (lit.)
SMILES string
CC(=O)\C=C(\C)O[Cu]O\C(C)=C/C(C)=O
InChI
1S/2C5H8O2.Cu/c2*1-4(6)3-5(2)7;/h2*3,6H,1-2H3;/q;;+2/p-2/b2*4-3-;
InChI key
QYJPSWYYEKYVEJ-FDGPNNRMSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A precursor for atomic layer deposition of copper oxide for all-oxide photovoltaics.
- A catalyst for the aziridination of styrene.
- A catalyst for Huisgen-Click reaction to synthesize 1,2,3-triazoles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Copper metal deposition processes are an essential tool for depositing interconnects used in microelectronic applications, giving group 11 (coinage metals: Copper, Silver, and Gold) an important place in atomic layer deposition (ALD) process development.
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure.
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service